Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.2 Joinville Abr./Jun. 2014
ORIGINAL RESEARCH ARTICLE
Histological analysis of cleaning capacity in apical third of flattened root canals with passive ultrasonic irrigation
Tiago Luís Boff I; Caroline Zamin I,II; Deborah Meirelles Cogo I; José Roberto Vanni I,III; Mateus Silveira Martins Hartmann I,III; Volmir João Fornari I,III
I Department of Endodontics, CEOM Dental School – Passo Fundo – RS – Brazil
II Community University of Chapecó Region – Chapecó – SC – Brazil
III IMED Dental School – Passo Fundo – RS – Brazil
ABSTRACT
Introduction and Objective: The aim of this study was to evaluate histologically the passive use of ultrasound for cleaning the apical portion of flattened root canal systems. Material and methods: The sample consisted of 20 extracted human mandibular incisors which were divided into two groups after being prepared with the rotary system Hero 642 up to size #45 surgical diameter: Group A – final irrigation with 4 ml of 2.5% sodium hypochlorite by the conventional technique using a syringe, and Group B – final irrigation with 4 ml of 2.5%, sodium hypochlorite divided into 1 ml amounts which were activated with the passive use of ultrasound for 15 seconds each time, generating a total activation period of 1 minute. Following, the teeth were subjected to morphometric analysis to evaluate the cleaning ability promoted in both groups. Results and Conclusion: Statistical analysis showed significant difference (p < 0.05) between the groups, with the passive ultrasonic irrigation resulting in cleaner canals.
Keywords: endodontics; root canal therapy; ultrasonic therapy.
Introduction
One of the goals of endodontic treatment is tooth preservation within the mouth, keeping it integrated into the masticatory system, thereby providing conditions for its repair allowing it to return to perform its functions normally. By knowing the relationship between the cleaning of the root canals system and the success of the endodontic treatment, the dentist must target on the highest possible cleaning when performing it 11,18.
Since the early 90s, the pattern has been automating the biomechanical preparation of the root canal system to reduce the time spent at this stage, keeping it more centered, increasing the chances of actually touching all the walls, avoiding risks of major defects in the apical region 14.
By achieving these requirements, the rotary Ni-Ti instrument is widely used in the treatment of root canals today, and it was the type of instrument selected for this study, although many scientific studies show that with the biomechanical preparation using Ni-Ti instruments it is not possible fully eliminate dentinal debris present inside the root canal system 12,13.
The anatomy complexity of root canal system is directly related to the quality of cleaning it. The root canals of lower incisors are often flattened at mesial-distal direction, and even with the use of Ni-Ti instruments, it is difficult to thorough cleaning the labial and lingual portions of these teeth 8.
To compensate for these cleaning failures, more resources are needed to improve the prognosis of endodontic treatment. In addition to the chemical action of the irrigant solutions and the physical action of the process of irrigation / aspiration that are part of the process of cleaning the root canal system 12, a passive irrigation using ultrasound can also be used.
The combination of conventional irrigation along with ultrasonic irrigation improves the elimination of bacteria and the smear layer around the root canal system, thereby contributing to higher rates of endodontic treatment success 1,11.
This study aimed to evaluate the effectiveness of using passive ultrasound irrigation after root canal instrumentation performed with rotary instruments Ni-Ti.
Material and methods
This quantitative experimental study consisted of a sample of 20 extracted human incisors selected according to the following criteria: presence of only one root canal, flattening of the proximal portion of root, intact root and fully formed apex. For this selection, the sample was previously imaged to exclude specimens that had more than one root canal, internal resorption and calcification. All teeth in the sample were obtained through donation after the signing of the Free and Clarified Consent Form, and the project was submitted and approved by the Ethics Committee in Research of the University Inga-UNINGÁ under protocol number #0002/10.
The teeth were stored into 10% formaldehyde solution until the time of use when they were washed in running water.
The X-ray was performed with Agfa Gevaert M2 3 x 4 cm film (Heraueus Kulzer, Hanau, Germany) with an exposure time of 0.5 seconds and object-film distance of 10 cm. For this purpose, it was used the x-ray machine Odontomax 70/7P (Astex Dental Equipment, São Paulo, SP, Brazil), with power of 70 kVp, current of 10 mA, open-locating cylinder of 20 cm and a total filtration of 5 mm aluminum. The films were processed manually and analyzed with X-ray light box and subsequently digitized.
The surgical access to the pulp chamber of the selected teeth, as well as wear and compensatory form of convenience were performed with diamond burs (KG Sorensen, São Paulo, SP, Brazil) at high-speed (Kavo Brazil, Joinville , SC, Brazil), and air / water cooling, aiming at a free and direct access to root canal. After the opening, the content of the pulp cavity was removed with size #15 K-files (Dentsply Maillefer, Ballaigues, Switzerland) and the apical foramen cleared with the same instrument. The canals were irrigated with disposable plastic syringe (Ultradent Products Inc., South Jordan, Utah, USA), using a Navitip needle (Ultradent Products Inc., South Jordan, Utah, USA) with a solution of 2.5% sodium hypochlorite (2.5% NaOCl, Pharmaceutical Rioquímica, Sao Jose do Rio Preto, São Paulo, Brazil), and its contents aspirated with siliconized Capillary tips (Ultradent Products Inc., South Jordan, Utah, USA), and then the complete drying was performed with absorbent paper points (Dentsply Maillefer, Ballaigues, Switzerland).
To perform the root canal preparation, X-Smart motor (Dentisply Maillefer, Ballaigues, Switzerland) set at a constant speed of 350 rpm, at clockwise direction and torque of 2 Newtons was used. The cervical roots were prepared with Protaper System size S1 and Sx instruments (Dentsply Maillefer, Ballaigues, Switzerland). To determine the working length, a K-file was carefully introduced into the root canal until its tip exceeded the apical foramen. This limit was analyzed on the operating microscope (M900; D.F. Vasconcellos S.A., São Paulo, SP, Brazil) with x10 magnification.
All root canals were prepared with size 642 Hero System (MicroMega, Besançon, France) in the following sequence: 20/.02 Hero at working length (WL), 25/.02 at WL, 25/.04 until resistance is encountered, 30/.02 at WL, 35/.02 at WL, 30/.06 until resistance is encountered, 40/.02 at n WL, and 45/.02 at WT. After every instrument change, the root canals were irrigated with 1 ml of 2.5% NaOCl and 1 ml of 17% EDTA (Pharmaceutical Industry Rioquímica, São José do Rio Preto, São Paulo, Brazil), used interchangeably, with disposable plastic syringe and Navitip needle for irrigation.
The prepared specimens were randomly divided into two groups: Group A – final irrigation with 4 ml of 2.5% NaOCl using the conventional technique with a syringe, and Group B – final irrigation with 4 ml of 2.5% NaOCl divided into 1 ml amounts that were ultrasonic passive activated (U.S. Jetsonic – GNATUS) using a size #15 K-file coupled to ultrasound device (A120 insert, GNATUS) at working length for 15 seconds each, resulting a total time of 1 minute of activation.
After preparation, the specimens were placed into properly identified individual flasks, containing 10% formalin solution for a period of 48 hours. After this period, the specimens were washed in running water and immersed in a solution of 10% trichloroacetic acid, renewed every 24 hours for a period of 15 days for decalcification. Completed this phase, the specimens were cut at 5 mm short of the apex, and these fragments were placed into plastic copings, marked their labial surface and properly identified, and washed in running water for 2 hours to remove acid residues before histological preparation.
The specimens were then subjected to histological processes and observed in an optical microscope (Nilkon®, Eclipse E 600, Japan) with x4 eyepiece 0:13 and x10/25 objective magnification was used yielding a final magnification of x40. Images were captured using Adobe Photoshop 5.1 software.
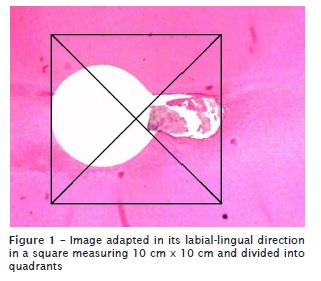
After, these images were released in Microsoft Power Point software, where, previously, the size of the slides was standardized so that the captured image adapted to the same extent (28.9 mm x 21.68 mm). The image was appropriate in its labial-lingual direction in a square measuring 10 mm x 10 mm and divided into quadrants, and then the counting of debris of each histological section (figure 1) could be realized and classified into scores. The scores were nominated and determined as follows:
Score zero: the absence of debris in all quadrants;
Score one: one quadrant with the debris presence;
Score two: two quadrants with the debris presence;
Score three: three quadrants with debris presence;
Score four: four quadrants with the debris presence.
Results
Preliminary tests were performed in order to determine the dust data from the two groups of teeth. The data presented normal distribution (p > 0.05), according to the Kolmogorov-Smirnov test. The variances were homogeneous (p > 0.05), according to the test of homogeneity of variances of Levene.
The analysis of the results obtained in these preliminary tests led to the conclusion that the sample distribution was normal, which led to perform the parametric analysis, and analysis of variance showed that there was a statistically significant difference between groups (p < 0.05).
Discussion
Because of the anatomical complexity found in the root canal system, i.e. the lower central incisors with a single root canal, which are often due to oval flattened mesial-distal root, adjuvant methods for cleaning are required to be used in endodontic treatment 11,18. In these cases, the instruments used in biomechanical preparation do not touch and remove the dentinal debris from various points of the inner portion of root canal, especially at the buccal and lingual walls 8. Wu and Wesselink 19 demonstrated the difficulty of instrumentation in oval canals, showing that in 40% of their sample there were areas not instrumented and incompletely filled.
Likely to other authors 10,13, this study was conducted with extracted human teeth. Although the use of natural samples makes difficult to control the variables of length, shape and diameter of root canals, the results may have greater clinical significance than those made in simulated canals in resin blocks. The surface of the simulated root canals is very different from that of natural dentin surface, which ultimately affect the bottom line. The natural sample reached a result more similar to the in vivo study, unlikely to the study of Townsend and Maki 17, who used simulated canals in resin blocks for your study. Lee et al. 7 considered that the porous nature of the dentin could set a different behavior of a synthetic material, such as those found in simulated root canals in glass or resin.
In order to standardize the sample, according to the study of Mancini et al. 9, we used only lower incisors with one root canal, flattening in the proximal root region, with fully formed apices and intact roots. The selection of teeth was performed with previous radiographs and ocular examination.
Similarly to the study of Gu et al. 3, the biomechanical preparation of the sample was carried out with rotary instrumentation system with Ni-Ti instruments to resemble everyday clinical practice. The use of Ni-Ti instruments is increasingly common in endodontic treatment because it reduces the time spent during the biomechanical preparation. The instrument works more centered in the canal maintaining its original format, increasing the chances of actually touching all the walls, decreasing major defects in the apical foramen as foramen transport. According Hülsmann et al. 5, the Ni-Ti rotary systems comply with the curvature of the root canal during the preparation, but fail to remove debris and smear layer in most cases.
In this study, ultrasound was used in order to stir the solution existing inside the root canal as well as made by Tasdemir et al. 16, not being provided directly by the tip of the ultrasound as in the studies of Gutarts et al. 4. Consideration should be given for the impossibility of using NaOCl inside the ultrasound device, which could damage it by doing so. As evidenced in the study of Zeltner et al. 20, and Kuah et al. 6, ultrasound increases the capacity for action of the irrigating solution used in endodontic treatment. This study used teeth with flattened root canals just to evaluate the effectiveness of ultrasound in removing the debris of the places where the instruments used in the biomechanical preparation are ineffective in cleaning the root canal walls.
After the biomechanical preparation of root canals, the methodology applied in this study was, the microscopic evaluation of the presence of debris remaining inside the root canal system. As in the study of Tanomaru-Filho et al. 15, histological sections of the apical third of roots were analyzed in an optical microscope. Images were captured using Adobe Photoshop 5.1 software. After these images were released in Microsoft Power Point and divided into quadrants, the counting of debris of each histological section could be performed and then classified into scores. The scores were nominated for the results of each section analyzed.
The results of this present study agree with those of Rödig et al. 13, who also used the ultrasound in a passive mode in the final irrigation with NaOCl. The studies have shown that the use of passive ultrasonic irrigation cleaned better the inner walls of the root canal. There was a statistically significant difference between groups, but in all groups, the debris still remained on the inner walls of root canals, as well as the study of Castagna et al. 2.
One must consider that in vitro scientific studies should approach the maximum that can be performed in the clinical treatment. For this, this study used only 4 ml of 2.5% NaOCl for irrigating root canals, unlikely to Passarinho-Neto et al. 12 who used 100 ml of sodium hypochlorite, which would be virtually unfeasible.
As shown by Wu and Wesselink 19, this study confirms that it is very difficult to remove all dirt from oval root canals, despite the passive aid of the ultrasound at their final irrigation. Although this method is efficient in cleaning the root canal system with flattened proximal surfaces, it still does not completely remove the debris from the root canal.
Conclusion
Through the results obtained from the methodology used in this study, it can be concluded that:
• The use of passive ultrasonic irrigation cleaned better the apical portion of root canals of the specimens used in this study;
• The instrumentation sequence associated with the technique and time of passive ultrasonic irrigation can be used clinically;
• Regardless of the techniques used in the study, debris remained inside the root canal system.
References
1. Cachovan G, Schiffner U, Altenhof S, Guentsch A, Pfister W, Eick S. Comparative antibacterial efficacies of hydrodynamic and ultrasonic irrigation systems in vitro. J Endod. 2013 Sep;39(9):1171-5. [ Links ]
2. Castagna F, Rizzon P, da Rosa RA, Santini MF, Barreto MS, Duarte MA et al. Effect of passive ultrassonic instrumentation as a final irrigation protocol on debris and smear layer removal – a SEM analysis. Microsc Res Tech. 2013 May;76(5):496-502.
3. Gu X, Mau C, Kern M. Effect of different irrigation on smear layer removal after post space preparation. J Endod. 2009;35:583-6.
4. Gutarts R, Nusstein J, Reader A, Beck M. In vivo debridment efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J Endod. 2005;31:166-70.
5. Hülsmann M, Gressmann G, Schäfers F. A comparative study of root canal preparation using FlexMaster and Hero 642 rotary NiTi. Int Endod J. 2003;36:358-66.
6. Kuah HG, Lui JN, Tseng PS, Chen NN. The effect of EDTA with and without ultrasonics on removal of the smear layer. J Endod. 2009;35:393-6.
7. Lee SJ, Wu MK, Wesselink PR. The effectiveness of syringe irrigation and ultrasonics to remove debris from simulated irregularities within prepared root canal walls. Int Endod J. 2004;37:672-8.
8. Malki M, Verhaagen B, Jiang LM, Nehme W, Naaman A, Versluis M et al. Irrigant flow beyond the insertion depth of an ultrasonically oscillating file in straight and curved root canals: visualization and cleaning efficacy. J Endod. 2012;38:657-61.
9. Mancini M, Armellin E, Casaglia A, Cerroni L, Cianconi L. A comparative study of smear layer removal and erosion in apical intraradicular dentine with three irrigating solutions: a scanning electron microscopy evaluation. J Endod. 2009;35:900-3.
10. Mayer BE, Peters OA, Barbakow F. Effects of rotary instruments and ultrasonic irrigation on debris and smear layer scores: a scanning electron microscopic study. Int Endod J. 2002;35:582-9.
11. Mozo S, Llena C, Forner L. Review of ultrasonic irrigation in endodontics: increasing action of irrigating solutions. Med Oral Patol Oral Cir Bucal. 2012;17:512-6.
12. Passarinho-Neto JG, Marchesan MA, Ferreira RB, Silva RG, Silva-Sousa YT, Sousa-Neto MD. In vitro evaluation of endodontic debris removal as obtained by rotary instrumentation coupled with ultrasonic irrigation. Aust Endod J. 2006;32:123-8.
13. Rödig T, Sedghi M, Konietschke F, Lange K, Ziebolz D, Hülsmann M. Efficacy of syringe irrigation, RinsEndo and passive ultrasonic irrigation in removing debris from irregularities in root canals with different apical sizes. Int Endod J. 2010;43:581-9.
14. Souza RA. Endodontia clínica. São Paulo: Santos; 2003.
15. Tanomaru-Filho M, Tanomaru JM, Leonardo MR, da Silva LA. Periapical repair after root canal filling with different root canal sealers. Braz Dent J. 2009;20:389-95.
16. Tasdemir T, Er K, Celik D, Yildirim T. Effect of passive ultrasonic irrigation on apical extrusion of irrigating solution. Eur J Dent. 2008;2:198-203.
17. Townsend C, Maki J. An in vitro comparison of new irrigation and agitation techniques to ultrasonic agitation in removing bacteria from a simulated root canal. J Endod. 2009;35:1040-3.
18. Van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40:415-26.
19. Wu MK, Wesselink PR. A primary observation on the preparation and obturation of oval canals. Int Endod J. 2001;34:137-41.
20. Zeltner M, Peters OA, Paqué F. Temperature changes during ultrasonic irrigation with different inserts and modes of activation. J Endod. 2009;35:573-7.
 Corresponding author:
Corresponding author:
Volmir João Fornari
Centro de Estudos Odontológicos Meridional (CEOM)
Rua Senador Pinheiro, n. 224 – Rodrigues
CEP 99070-220 – Passo Fundo – RS – Brasil
E-mail: ceom@ceompf.com.br
Received for publication: November 25, 2013
Accepted for publication: December 20, 2013













