Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.2 Joinville Abr./Jun. 2014
ORIGINAL RESEARCH ARTICLE
Comparative analysis of four cleaning methods of endodontic files
Bárbara Guandalini I; Ivana Vendramini I; Denise Piotto Leonardi I; Flávia Sens Fagundes Tomazinho I; Paulo Henrique Tomazinho I
I Department of Dentistry, Positivo University – Curitiba – PR – Brazil
ABSTRACT
Introduction: Due to the size and design of endodontic files, these instruments have been considered one of the most difficult to clean among all dental instruments. The debris maintenance within the sulcus prevents the effective sterilization and may compromise the disinfection of root canal systems in endodontic therapy. However, there is neither a method nor technique that standardized the cleaning of these instruments. Objective: To evaluate the cleaning ability of four techniques used in dentistry. Material and methods: For this purpose, 30 new size #40 Flexofile were used for the preparation of the canals of mandibular molars of pigs. After instrumentation, the contamination and the presence of debris in the sulcus was confirmed and the files were randomly divided into four groups: control group (without cleaning), group 1 (enzymatic detergent + manual brushing with nylon bristle brush), group 2 (ultrasound + enzymatic detergent), group 3 (ultrasound + water) and group 4 (gauze embedded in 70% alcohol). Next, all files were photographed and photographs were printed at high quality. The spirals containing debris were counted. Results: Manual cleaning with enzymatic detergent and nylon bristle brush, ultrasound with either water or detergent showed the best cleaning capacity in which respectively 100%, 98.9% and 96.2%, of the spirals were free of debris. Cleaning with alcohol and gauze proved to be ineffective, showing debris in more than 40% of the spirals by visual analysis. In control group, 91% of the spirals presented debris. It can be concluded that the association between manual and ultrasound cleaning may be promising in ensuring a cleaning protocol for endodontic files cleaning.
Keywords: biosafety; Endodontics; cleaning method.
Introduction
The endodont ic files are composed with stainless steel or nickel-titanium and display their active point with different cross-sectional designs, forming their spirals 6. They have been considered as critical instruments because they penetrate within subepithelial tissues, reaching the vascular system 7. The action of the file is to scratch the root canal walls to detach dentin portions and remove it towards outside the canals. Accordingly, debris are left along their spirals. Because of the design, presenting angles between the spirals and the long axis of the files, the authors report great difficulty in the cleaning of these instruments 13,21.
The lack of standardization of the cleaning methods of the endodont ic f i les results in many controversies related to the most effective decontamination protocol, thus raising great interest in this subject 13. Queiroz 15 emphasized that cleaning techniques aiming at eliminating the debris within the file spirals must be used to prevent the disinfection and sterilization process. On the other hand, Sousa 19 conducted a comparative study on four cleaning methods. The author evaluated the cleaning through dry gauze, flask with sponge, and flask with gauze. The most effective method was the sponge. Figueiredo and Sydney 4 evaluated five techniques: tap water, brush and soap; ultrasound and brushing; brushing and ultrasound; and only ultrasound. They found that although all methods presented debris, the most satisfactory results were found in the group in which ultrasound was used followed by brushing. Manual washing was the least effective technique. Reiss-Araújo et al. 16 evaluated the cleaning technique of endodontic files applied by undergraduates. The students were divided into three groups: 1) cleaning by the method adopted by the dental school (non standardized), 2) cleaning with ultrasound, brushing, and soap and 3) none cleaning technique of endodontic files. After the photomicrographic analysis with stereomicroscopy, the authors concluded that ultrasound was the most effective technique and the cleaning protocol adopted by the dental school was ineffective for a correct sterilization of the instruments.
The maintenance of the aseptic chain is essential for a favorable prognosis in endodontic treatment. To reach endodontic success, one ought to clearly comprehend not only the root canal morphology, but also its variations 18. If an endodontic file exhibits debris within its spirals, even after sterilization, it may carry the remnants towards inside root canal. This debris may create a protection barrier for microorganisms. Endodontic files autoclaved with organic matter within the spirals may jeopardize the sterilization process because the organic matter protect the microorganism against unsaturated steam and prevent thermocoagulation of the microbial structures accounting for cellular death and the instrument sterilization. The presence of organic matter and residues in endodontic instruments may lead to cross-contamination and, consequently, treatment failure 11.
Because most of endodont ists reuse the endodontic files, their cleaning and sterilization is mandatory for treatment success.
The aim of this study was to evaluate different cleaning methods of endodontic files through the analysis of debris by visual method of enlarged photographs.
Material and methods
The sample was composed by 30 new size #40 endodontic files (Maillefer, Ballaigues, Switzerland). Root canal instrumentation was performed in porcine molars in dissected fresh pig. The apparent tooth length was determined with the aid of radiographs. Each file was used to prepare only one canal by using ten repetitive ¼ turn clockwise movements against the root canal walls.
After the instrumentation of each root canal, the files were randomly divided into four groups (n = 6): control (no treatment), group 1 (enzymatic detergent + manual brushing), group 2 (ultrasound + enzymatic detergent), group 3 (ultrasound + water) and group 4 (gauze with alcohol).
Following the use of control group files, they were not submitted to any cleaning process. Then, they were individually stored in test tubes with screw cap, properly identified.
Group 1 files (enzymatic detergent + manual brushing), after their use, were immersed in a flask containing enzymatic detergent (Riozyme, Rioquimica, São José do Rio Preto, Brazil), diluted in water for 5 minutes, according to the manufacturer's recommendations. Twenty instruments (tweezers, explores, mirrors, curettes, glass plates etc.), all purposefully contaminated with debris from the porcine mandibles, were put together with the endodontic files so that the brushing and cleaning of the files were not evidenced. All instruments were washed by soft nylon bristle brushes under continue flow and dried with air jet. The files were stored in test tubes with screw cap, properly identified. The washing of both the files and instruments were executed by a volunteer who did not know the aims of the research.
After the use, the group 2 files (ultrasound + enzymatic detergent) were placed in ultrasound device (Cristófoli, Campo Mourão, Brazil) with water and enzymatic detergent, for five minutes according to the manufacturer's recommendations. Elapsed this period, the files were washed under continuous water flow, dried with air jet and stored in a test tube with screw cap, properly identified.
Group 3 files (ultrasound + water) after use were put in the ultrasound device with water for five minutes according the manufacturer's recommendations. Elapsed this period, they were washed in continuous water flow dried with air jet and stored in a test tube with screw cap, properly identified.
After the use, group 4 files (gauze with alcohol) were cleaned with gauze embedded with 70% alcohol by three repetitions of the cleaning movement (apprehend the active part of the file with the gauze and pull it), followed by air jet and stored in test tube with screw cap, properly identified.
All stored files were taken to the Microbiology Laboratory of the Positive University, where photographs were taken by digital camera (Nikon D90, Nikon, Tokyo, Japan) with 100 mm macro lens (Sigma, Rödermark, Germany) and round flash (Nikon, Tokyo, Japan). The photographs were printed at color high definition (HP LaserJet 1020, Hewlett Packard, Palo Alto, USA), on size A4 photographic paper. The results were evaluated through visual analysis of the amplified photographs of the endodontic files by counting the spiral presenting either debris or organic matter.
Results
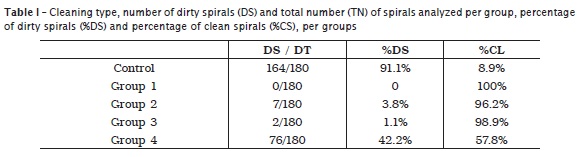
The results of the count of either the inorganic debris or organic matter are displayed in table 1.
Discussion
Many studies reported on the important role of the cleaning of endodontic files before, during and after their use 2,4,8,9,12,14,17. All these stages assure the proper sterilization, so that endodontic files can be safely reused, contributing for endodontic success. Some authors defend the exclusively manual cleaning 2,14; others claimed that the debris within the files are cleaned by ultrasound 3,9,12 while other emphasized the use of both techniques, that is, the use of ultrasound associated with manual cleaning 4,8,17.
Evidences have supported that endodontic files are difficult to clean and can carry significant remnants after their washing 5. The sterilization process comprises the destruction or removal of all life forms within a material and it is the most important step of infection control. A partial sterilized instrument does not exist; the instrument is or is not sterilized. However, it should be emphasized that the sterilization effectiveness depends on the previous preparation of the instrument. These preparations have been divided into pre-washing, washing, drying, storage and sterilization 7.
The aim of this present study was to conduct a comparative study on four cleaning methods of endodontic files, to evaluate the cleaning effectiveness of these methods regarding to the presence or absence of visible debris in the file spirals. For this purpose, porcine teeth were used to mimic the instrumentation of root canals, aiming to the debris retention (organic matter or inorganic debris) on the file spirals. This experimental model was very effective in retaining debris on the file spirals and should be considered as an option for further studies with similar aims.
Linsuwanont et al. 10 proposed a methodology for the cleaning of rotary nickel-titanium instruments by associating moist storage, brushing followed by immersion in 1% sodium hypochlorite. The authors affirmed that by using this technique, 100% of the file spirals were cleaned and free of debris. On the other hand, Reiss-Araújo et al. 16 alert that the manual cleaning itself is subject to human error, such as omission or failure in cleaning itself. The authors still emphasized that nylon bristles did not penetrate in the angle formed by the spirals and body of most of the endodontic files. Aasim et al. 1 defended the use of automatized cleaning such as the ultrasound device especially for instruments difficult to clean, as endodontic files. The results of this present study showed that manual cleaning was capable of removing 100% of the debris within the file spirals through visual analysis. Ultrasound associated with either enzymatic detergent or water alone cleaned 96.2% and 98.9% of the spirals, respectively. These results evidenced the excellent cleaning capacity of the ultrasonic mechanical action promotion on these instruments, corroborating previous studies 1,3,9,12.
By empirically observation, we noted that the undergraduates of Positivo University cleaned the endodontic files with gauze and 70% alcohol. This observation justified the inclusion of this method in this present study (group 4). In group 4, after the analysis of the enlarged photographs, it was observed that 40% of the spirals remained dirty with debris or organic matter from the pulp tissues of porcine teeth. Although some authors 9,19,20 affirmed that regardless of the cleaning technique the sterilization process is effective, this assumption is denied by other studies 13 discussing the necessity of the cleaning and reduction of the debris and microorganism amount on the instrument surface to improve the effectiveness of the sterilization process.
Aasim et al. 1 showed that the ultrasound is able to remove all debris of the instruments during an interval from 5 to 10 minutes and did not observed changes in the action time longer than 10 minutes to a maximum of one hour. On the other hand, according to the result of this present study, spirals with debris were seen even in the groups submitted to ultrasonic cleaning. Accordingly, it seems licit to propose the following protocol: initial manual cleaning followed by ultrasound with or without enzymatic detergent. Thus, we associated the advantage of the debris removal by manual cleaning with the cleaning capacity of ultrasound, which can reach sites where manual cleaning did not act, such as the angles formed between the spiral and the body of the instrument.
This study drew attention for a process many times neglected during clinical endodontic practice – the cleaning steps previous to sterilization.
Despite of the limitations of this study, such as the microscopic analysis through either optical or electronic microscope; and the absence of microbiological test which could have evidenced a grater or smaller microbiological contamination previous and/or after the sterilization process, it was observed the viability of the use of porcine teeth in this study type because a condition similar to that of human teeth was found, that is, the presence of pulp tissue and dentine debris within the spirals of the endodontic files.
Conclusion
The manual cleaning of endodontic files with enzymatic detergents and nylon bristle brush was effective in cleaning the debris within file spirals through visual analysis. It is suggested a protocol comprising the manual cleaning followed by ultrasound for these instruments.
References
1. Aasim SA, Mellor AC, Qualtrough AJE. The effect of pre-soaking and time in the ultrasonic cleaner on the cleanliness of sterilized endodontic files. Int Endod J. 2006;39:143-9. [ Links ]
2. Alvarez S. Endodontia clínica. 2. ed. São Paulo: Santos; 1991. 3. Deus QD. Endodontia. 5. ed. Rio de Janeiro: Medsi; 1992.
4. Figueiredo JAP, Sydney GB. Eficácia das técnicas de limpeza de instrumentais endodônticos retentivos. Rev Paranaense Odontol. 1997;2(2): 1-12.
5. Gill DS, Tredwin CJ, Gill SK, Ironside JW. The transmissible spongiform encephalopathies (prion diseases): a review for dental surgeons. Int Dent J. 2001;51:439-46.
6. Goldberg F, Soares J. Endodontia – técnicas e fundamentos. 2. ed. Porto Alegre: Artmed; 2011.
7. Guandalini SL, Melo NSF, Santos ECP. Biossegurança em Odontologia. 2. ed. Curitiba; 1999.
8. Haïkel Y, Serfaty R, Bleicher P, Lwin TT, Allemann C. Effects of cleaning, disinfection, and sterilization procedures on the cutting efficiency of endodontic files. J Endod. 1996;22(12):657-61.
9. Ingle JI, Taintor JF. Endodontia. Rio de Janeiro: Guanabara Koogan; 1989. 730 p.
10. Linsuwanont P, Parashos P, Messer HH. Cleaning of rotary nickel-titanium endodontic instruments. Int Endod J. 2004;37:19-28.
11. Miller CH. Sterilization: disciplined microbial control. Dent Clin Noorth Am. 1991;35(2):339-55.
12. Murgel CAF, Walton RE, Rittman B, Johnson AA. A comparison techniques for cleaning endodontic files after usage: a quantitative scanning electron microscope study. J Endod. 1990;16(5):214-7.
13. Oliveira EPM, Filippini HF, Troian HC, Melo TAF. Análise das condições de esterilidade das limas endodônticas utilizadas pelos alunos de graduação nos três cursos de Odontologia da ULBRA/RS. Stomatos. 2006;12(23):35-40.
14. Paiva JG, Antoniazzi JH. Endodontia: bases para prática clínica. São Paulo: Artes Médicas; 1988. p. 463-80.
15. Queiroz MLP. Avaliação comparativa de eficiência de diferentes técnicas empregadas na limpeza de limas endodônticas. Canoas. Mestrado [Dissertação] – Universidade Luterana do Brasil; 2001.
16. Reiss-Araújo CJ, Araújo SS, Albuquerque DS, Rios MA, Portella ML. Limpeza em limas endodônticas pós-uso e pré-esterilização. RGO. 2008;56(1):17-20.
17. Rossetini SMO. Contágio no consultório odontológico: como entender e prevenir. São Paulo: Santos; 1985. p. 72-89.
18. Simşek N, Keleş A, Bulut ET. Unusual root canal morphology of the maxillary second molar: a case report. Case Rep Dent. 2013;2013.
19. Sousa SMG. Análise comparativa de quatro métodos de limpeza de limas endodônticas durante o transoperatório: estudo pela microscopia eletrônica de varredura. Bauru. Dissertação [Mestrado] – Universidade de São Paulo; 1994.
20. Viegas APK. A importância da limpeza de limas endodônticas contaminadas no processo de esterilização. Canoas. Dissertação [Mestrado] – Universidade Luterana do Brasil; 2005.
21. Weine FS. Tratamento endodôntico. 5. ed. São Paulo: Santos; 1998. .
 Corresponding author:
Corresponding author:
Paulo Henrique Tomazinho
Departamento de Odontologia, Universidade Positivo
Rua Professor Pedro Viriato Parigot de Souza, n. 5.300 – Campo Comprido
CEP 81280-330 – Curitiba – PR – Brasil
E-mail: paulo@tomazinho.com.br
Received for publication: November 10, 2013
Accepted for publication: December 11, 2013













