Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.3 Joinville Jul./Set. 2014
ORIGINAL RESEARCH ARTICLE
Evaluation of dentinal permeability reduction provided by different desensitizing treatments
Fabio Antonio Piola Rizzante I; Rafael Massunari Maenosono I; Regina Guenka Palma-Dibb II; Marco Antonio Hungaro Duarte I; Sérgio Kiyoshi Ishikiriama I
I Department of Restorative Dentistry, Endodontics and Dental Materials, Bauru School of Dentistry, University of São Paulo – Bauru – SP – Brazil
II Department of Restorative Dentistry, Ribeirão Preto School of Dentistry, University of São Paulo – Ribeirão Preto – SP – Brazil
ABSTRACT
problem at dental offices and new approaches may be developed. Objectives: The authors studied different desensitizing treatments and their efficacy in reducing dentinal permeability and dentinal tubules opening. Material and methods: One hundred bovine incisors roots had their buccal surface flattened and treated by 3 applications of each desensitizing agent, following the respective groups (n = 10). After treated, 7 specimens of each group were prepared for a 0.5% basic fuchsin permeability test and the other 3 specimens were prepared to SEM qualitative analysis. The permeability test specimens were sectioned with a diamond saw in order to evaluate the stained and unstained areas. Kruskall Wallis statistical analysis was performed (p < 0.05). Results: Colgate Pró-Alívio paste and toothpaste, diode and Nd:YAG Lasers, GHF, Sensi Active, Oxagel and 2% Desensibilize promoted a significant permeability reduction when compared with the respective control groups (p < 0.05). Comparing the mean permeability differences between the different groups after the treatments, Oxagel and Nd:YAG were better than 0.2% Desensibilize group. Conclusion: None of the treatments may be considered 100% effective in treating dentinal hypersensitivity since a partial reduction of the permeability was observed.
Keywords: dentin sensitivity; sodium fluoride; sensitivity; root; preventive dentistry.
Introduction
In recent decades, a significant reduction in the incidence of teeth decay was observed 27,33. Thus, the teeth have remained longer in the mouth, which makes them more susceptible to other types of non-carious lesions such as gingival recession and tooth wear which may, in some cases, cause dentin hypersensitivity 1,2,35,36. The prevalence of hypersensitivity varies widely (from 8% to 57%) depending on the population, methodology and resources used for diagnostic 3,36,42.
Dentin hypersensitivity has a multifactorial etiology and can be described clinically as an acute pain that occurs in response to thermal (hot or cold), chemical (acid fruits, spicy foods, sugar and salt), mechanical (brushing) and evaporative stimuli (air jets) applied to the exposed dentin due to the presence of opened dentinal tubules. The exposure of dentin to the oral environment may occur due to processes as gingival recession and root erosion, abrasion or attrition, as well as surgical and non-surgical periodontal treatments 3,5,8,22,32,42.
The most widely accepted theory to explain dentinal hypersensitivity is the "Hydrodynamic Theory" from Brännström 7 that provides a plausible explanation for the hypersensitivity manifestation in which the motion of dentinal tubules internal fluids are able to stimulate nervous cells present in the pulp tissue, leading to a painful sensation 8,30.
The treatment of dentinal hypersensitivity has as goal pain remission, often by topic application of desensitizing agents, anti-inflammatory agents, agents that block the neuronal response, root coverage procedures through periodontal plastic surgery and, most currently, high and low input laser irradiation 5,11,43.
Although there are different therapies for the treatment of dentin hypersensitivity, the main challenge is to find a substance or treatment that effectively eliminates the pain and does not relapse, which unfortunately is still not available 28.
So, it is fundamental to have comparative studies of different treatments and products, considering their dentinal tubules physical obliteration efficiency and the associated dentin permeability reduction.
Material and methods
Experimental design
The experimental design presented one variation factor (treatment), divided in ten levels. The quantitative response variable was percentage of infiltrated area, measured by imaging software. The sample size was 100 bovine incisors, divided into ten groups (n = 10).
Specimen preparation
One hundred bovine incisors had their roots separated from the respective crowns. The root lingual surfaces were flattened with 320 grit SiC sandpaper (Extec. Corp., Enfield, USA) and after achieving a flat surface, the specimens were fixed in acrylic discs using viscous wax, with the buccal surface upwards. This surface was also flattened and polished using 320, 600 and 1200 grit SiC sandpaper, at low speed (Figure 1). Specimens were stored in 0.1% thymol solution after extraction for a maximum time period of 3 months. All specimens were prepared at the same time and stored at 4ºC deionized water until used (treatment applications).
The specimens were randomly divided into 10 groups (n = 10), with 3 applications of different desensitizing treatments. Seven specimens of each group were used for dye infiltration test and the others were used for visual analysis in scanning electron microscopy (SEM).
Each flattened surface was marked in the center with a ¼ steel drill at low speed in order to delimit the test and control areas. EDTA pH 7.5 was applied (1 minute) over the f lattened surface, with a KG microbrush (Kg Sorensen, Cotia, SP, Brazil), in order to remove debris allowing a wide-open dentinal tubules aperture. Half of the surface was protected with a lightcured gingival barrier (Top-Dam, FGM, Joinvile, SC, Brazil), based on the previously center mark. On the other half (non-protected) the treatment was performed according to the guidelines of the respective manufacturer (Table 1 and Figure 2).

Considering the laser groups, a 30 mm² area (5.0 X 6.0 mm) was delimited using the gingival barrier, half of this area (15 mm2 – 2.5 X 6.0 mm) was protect with gingival barrier (control area) and the unprotected test area was irradiated as follows:
- Nd:YAG laser: 1064nm wave-length, 300 μm fiber diameter, 0.5W power output, 10 Hz frequency, long pulse and entire area scanning (30s) in contact mode;
- Diode laser: 970nm wave-length, 300 μm fiber diameter, 0.5W power output, 10 Hz frequency, long pulse and entire area scanning (30s) in contact mode.
After the treatments, the gingival barrier was removed of the specimens' surface.
Permeability tests
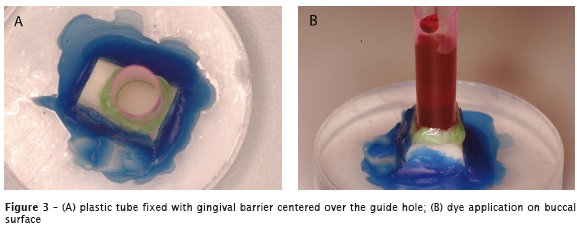
In order to realize the permeability test, 0.1mL of 0.5 % basic fuchsin was placed in contact with the buccal surface for 4 hours, using a 5mm diameter plastic tube centered over the guide hole, hold steady with use of the gingival barrier (Figure 3A and 3B).
After the dye infiltration time, the plastic tube and gingival barrier were removed and the surface was washed with air-water spray for 2 minutes.
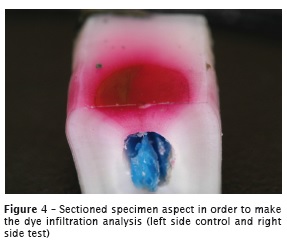
The stained specimens were taken to the cut machine in order to perform a mesiodistal section, also based on the guide hole. In sectioned specimens was possible to visualize the test and control surfaces (Figure 4).
The image of each half was captured with a professional digital camera (Nikon D100, Japan). The digital images were transferred to UTHSCSA Image Tools version 3.0 software, where the total area (from flattened surface to root canal as vertical length) and the dye leakage area (test and control sides) were quantified, allowing to calculate the percentage of dye penetration in each specimen.
Scanning Electron Microscopy
Three randomly selected specimens of each group were processed for qualitative analysis. The specimens were fixed on a metallic stub with their treated surfaces facing up. After dried in an desiccator and metallized, the samples were analyzed in a scanning electron microscope JSMT220a (Jeol, Tokyo, Japan) with 15 kV and 1000X magnifying. Digital photomicrographs, from both test and control sides, were analyzed qualitatively illustrating the behavior of each treatment.
Statistical analysis
The results of each group were obtained from the percentage of infiltrated (stained) area versus the total area. Since the means did not reach the normality test, a non-parametric Kruskal-Wallis test was applied (p < 0.05).
Results
Permeability
The infiltration observed (% ± SD) in all groups after 3 applications of the desensitizing treatment is shown in table 2 (test and control areas values) and table 3 (test and control mean differences).
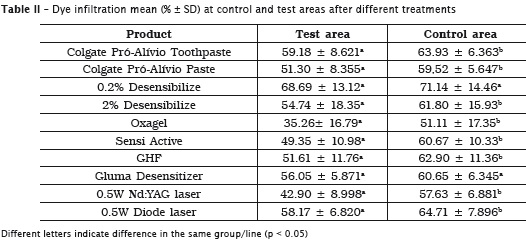
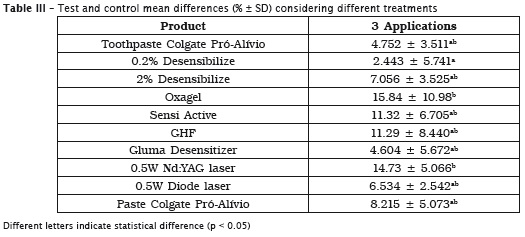
Colgate Pró-Al ívio paste (in of f ice) and toothpaste, diode and Nd:YAG Lasers, GHF, Sensi Active, Oxagel and 2% Desensibilize promoted a significant permeability reduction than the respective control groups (p < 0.05). Comparing the mean permeability differences between the different groups after the treatments, Oxagel and Nd:YAG were better than 0.2% Desensibilize group.
Scanning Electronic Microscopy
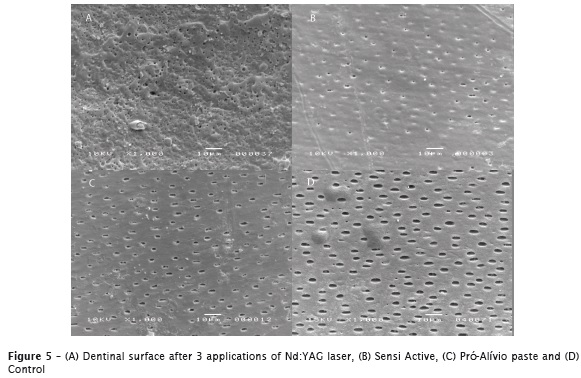
In general, a superficial deposit and consequently reduction in diameter and obliteration of the dentinal tubules could be observed, at varying degrees, but with the presence of some open dentinal tubules. Considering the desensitizing agents and the lasers, different levels of superficial deposit were observed; when considering the Nd:YAG laser group, an irregular surface with mosaic-like structures was observed, compared with the wide open tubules observed in the control groups (figures 5A, 5B, 5C and 5D).
Discussion
Dentin hypersensitivity has been the subject of many studies that have evaluated different strategies and products for its treatment 5,11,43 and the use of bovine dentin near to the cementum-enamel junction consists in a suitable substitute of the human dentin considering the permeability and SEM analysis 38. Among the tested products in this study, it is worth mentioning the different action mechanisms. The GHF and Desensibilize are NaF based products and act through the formation of a CaF2 layer on the surface promoting a dentinal permeability reduction through the obliteration of exposed dentinal tubules 12,13,25. It is important to say that GHF also has glutaraldehyde in its composition, which may act as an active component in desensitizing. In the present study, a significant reduction in dentin permeability could be observed after 3 applications of these products.
The Oxagel and Sensi Active are potassium oxalate based products that also act by depositing crystal-like structures within the lumen of the tubules 5. These crystals are formed after active substance penetration within the tubules resulting in insoluble calcium oxalate crystals formation 6,15,17,19,31. The precipitates occur within the dentinal tubules, forming crystals that extend 15 μm depth and with various dimensions that allow different degrees of tubules occlusion 10. This fact can explain the results of the present study in which, even with a superficial deposit not as great as in some treatments, the permeability test showed an statistically significant reduction in dentin permeability.
The Gluma Desensitizer is an aqueous solution containing 5% glutaraldehyde and 35% HEMA. It has been suggested that its action mechanism is based on the dentinal tubules occlusion through reaction with plasmatic dentinal fluid, which would lead to the formation of a thin resin layer of about 1 μm thickness (due to the HEMA polymerization) that can obliterate the dentinal tubules 24,40. In the present study, Gluma showed no significant reduction in dentin permeability and these results are consistent with a clinical study where no reduction in dentin hypersensitivity was observed before one week after agent application 24.
Pastes and toothpastes containing desensitizing agents has also been widely researched and produced. In the products tested in this study, these agents differ by the presence of 1.10% sodium monofluorophosphate, present in the toothpastes. The arginine and calcium carbonate work together in order to accelerate the dentinal tubules natural obliteration mechanisms by "dentin-like" mineral deposits containing calcium phosphate within dentinal tubules 34. In the present study, a significant reduction of dentin permeability was observed after three applications of both products, but in photomicrographs a great number of opened dentinal tubules could still be observed.
Another treatment used to relief dent in hypersensitivity by physical obstruction of the tubules is high-intensity laser irradiation on hypersensitive dentine. According to some authors, the laser consists in an easy application method and also painless, with a fast action 9,14,18,20. According with Dilsiz et al. 2009 10 there are two types of lasers: the high (e.g. Nd: YAG) and low level (e.g. diode laser) that can in some models, operate at high intensity.
The Nd: YAG laser has been used for the reduction of dentin hypersensitivity as it induces an occlusion or narrowing of the dentinal tubules, leading to a pain relief 14,21,23. Dentin is fused after a brief laser exposure (melting), whereas it re-solidifies in the form of a glazed and non-porous surface 4. In the present study, all irradiated samples showed the same surface changes with irregular mosaic-like structures with a significant reduction in dentin permeability. Similar results also were observed by other authors 16,23,37,39.
In the present study, the diode laser also promoted a signi f icant decrease in dent i n permeability. In photomicrographs a reduction in the number and diameter of dentinal tubules could be observed. Umberto et al. 2012 41, also observed a significant reduction in dentin hypersensitivity after 980nm diode laser irradiation.
It is also unclear whether permeability must be completely stopped before sensitivity can be treated, considering this, it is important to say that some substances may also have a desensitizing effect observed only "in vivo", such as Desensibilize that has also potassium nitrate in its formula, which acts as a neural depolarizer; Sensi Active and Oxagel, based on potassium oxalate, which can inhibit the transmission of nerve impulses 26. Besides these, the low-level lasers can also interact with the pulp tissue inducing a biomodulatory effect that increases odontoblastic metabolic activity enhancing the production of tertiary dentin and can also interfere in the sodium pump reducing intra dental nervous response 29,44.
Even with the limitations of this study it has been concluded that none of the treatments may be considered 100% effective in treating dentinal hypersensitivity since a partial reduction of the permeability was observed.
References
1. Abdullah Gh A, Mansour Ali SA. Statistical analysis of the prevalence, severity and some possible etiologic factors of gingival recessions among the adult population of Thamar city, yemen. RSBO. 2011;8(3):305-13. [ Links ]
2. Addy M, Shellis RP. Interaction between attrition, abrasion and erosion in tooth wear. Monogr Oral Sci. 2006;20:17-31.
3. Ahmed TR, Mordan NJ, Gilthorpe MS, Gillam DG. In vitro quantification of changes in human dentine tubule parameters using SEM and digital analysis. J Oral Rehabil. 2005 Aug;32(8):589-97.
4. Aranha ACC, Domingues FB, Franco VO, Gutknecht N, Eduardo CD. Effects of Er : YAG and Nd : YAG lasers on dentin permeability in root surfaces: a preliminary in vitro study. Photomedi Laser Surg. 2005 Oct;23(5):504-8.
5. Arrais CA, Chan DC, Giannini M. Effects of desensitizing agents on dentinal tubule occlusion. J Appl Oral Sci. 2004 Jun;12(2):144-8.
6. Assis JS, Rodrigues LK, Fonteles CS, Colares RC, Souza AM, Santiago SL. Dentin hypersensitivity after treatment with desensitizing agents: a randomized, double-blind, split-mouth clinical trial. Braz Dent J. 2011;22(2):157-61.
7. Brannstrom M. Dentin sensitivity. Arsb Goteb Tandlak Sallsk. 1964:15-35.
8. Brannstrom M, Linden LA, Astrom A. The hydrodynamics of the dental tubule and of pulp fluid. A discussion of its significance in relation to dentinal sensitivity. Caries Res. 1967;1(4):310-7.
9. Ciaramicoli MT, Carvalho RC, Eduardo CP. Treatment of cervical dentin hypersensitivity using neodymium: Yttrium-aluminum-garnet laser. Clinical evaluation. Lasers Surg Med. 2003;33(5):358-62.
10. Dilsiz A, Canakci V, Ozdemir A, Kaya Y. Clinical evaluation of Nd:YAG and 685-nm diode laser therapy for desensitization. Photomed Laser Surg. 2009 Dec;27(6):843-8.
11. Frechoso SC, Menendez M, Guisasola C, Arregui I, Tejerina JM, Sicilia A. Evaluation of the efficacy of two potassium nitrate bioadhesive gels (5% and 10%) in the treatment of dentine hypersensitivity. A randomised clinical trial. J Clin Periodontol. 2003 Apr;30(4):315-20.
12. Ganss C, Klimek J, Brune V, Schurmann A. Effects of two fluoridation measures on erosion progression in human enamel and dentine in situ. Caries Res. 2004 Nov-Dec;38(6):561-6.
13. Ganss C, Klimek J, Starck C. Quantitative analysis of the impact of the organic matrix on the fluoride effect on erosion progression in human dentine using longitudinal microradiography. Arch Oral Biol. 2004 Nov;49(11):931-5.
14. Gelskey SC, White JM, Pruthi VK. The effectiveness of the Nd:YAG laser in the treatment of dental hypersensitivity. J Can Dent Assoc. 1993 Apr;59(4):377-8, 383-6.
15. Gillam DG, Mordan NJ, Sinodinou AD, Tang JY, Knowles JC, Gibson IR. The effects of oxalatecontaining products on the exposed dentine surface: an SEM investigation. J Oral Rehabil. 2001 Nov;28(11):1037-44.
16. Goodis HE, White JM, Marshall Jr GW, Yee K, Fuller N, Gee L et al. Effects of Nd: and Ho:yttriumaluminium- garnet lasers on human dentine fluid flow. Arch Oral Biol. 1997 Dec;42(12):845-54.
17. Greenhill JD, Pashley DH. The effects of desensi t izing agents on the hydraul i c conductance of human dentin. J Dent Res. 1981 Mar;60(3):686-98.
18. Gutknecht N, Moritz A, Dercks HW, Lampert F. Treatment of hypersensitive teeth using neodymium:yttrium-aluminum-garnet lasers. J Clin Laser Med Surg. 1997;15(4):171-4.
19. Kolker JL, Vargas MA, Armstrong SR, Dawson DV. Effect of desensitizing agents on dentin permeability and dentin tubule occlusion. J Adhes Dent. 2002 Fall;4(3):211-21.
20. Lan WH, Liu HC. Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Laser Med Surg. 1996 Apr;14(2):89-92.
21. Lee BS, Lin CP, Lin FH, Lan WH. Ultrastructural changes of human dentin after irradiation by Nd : YAG laser. Lasers Surg Med. 2002;30(3):246-52.
22. Leme AFP, dos Santos J, Giannini M, Wada RS. Occlusion of dentin tubules by desensitizing agents. Am J Dent. 2004 Oct;17(5):368-72.
23. Liu HC, Lin CP, Lan WH. Sealing depth of Nd: YAG laser on human dentinal tubules. J Endod. 1997 Nov;23(11):691-3.
24. Lopes AO, Correa Aranha AC. Comparative evaluation of the effects of Nd:YAG laser and a desensitizer agent on the treatment of dentin hypersensitivity: a clinical study. Photomed Laser Surg. 2013 Mar;31(3):132-8.
25. Magalhaes AC, Levy FM, Rizzante FA, Rios D, Buzalaf MA. Effect of NaF and TiF(4) varnish and solution on bovine dentin erosion plus abrasion in vitro. Acta Odontol Scand. 2012 Mar;70(2):160-4.
26. Markowitz K, Bilotto G, Kim S. Decreasing intradental nerve act ivi ty in the cat wi th potassium and divalent-cations. Arch Oral Biol. 1991;36(1):1-7.
27. Narvai PC, Frazao P, Roncalli AG, Leopoldo F, Antunes J. Dental caries in Brazil: decline, polarization, inequality and social exclusion. Rev Panam Salud Publica – Pan Am J Public Health. 2006 Jun;19(6):385-93.
28. Naylor F, Correa Aranha AC, De Paula Eduardo C, Arana-Chavez VE, Pita Sobral MA. Micromorphological analysis of dentinal structure after irradiation with Nd : YAG laser and immersion in acidic beverages. Photomed Laser Surg. 2006 Dec;24(6):745-53.
29. Orchardson R, Whitters CJ. Effect of HeNe and pulsed Nd:YAG laser irradiation on intradental nerve responses. Lasers Surg Med. 2000;26(3):241-9.
30. Pashley DH. Mechanisms of dentin sensitivity. Dent Clin North Am. 1990 Jul;34(3):449-73.
31. Pashley DH, Carvalho RM, Pereira JC, Villanueva R, Tay FR. The use of oxalate to reduce dentin permeability under adhesive restorations. Am J Dent. 2001 Apr;14(2):89-94.
32. Pashley DH, O'Meara JA, Kepler EE, Galloway SE, Thompson SM, Stewart FP. Dentin permeability. Effects of desensitizing dentifrices in vitro. J Periodontol. 1984 Sep;55(9):522-5.
33. Petersson HG, Bratthall D. The caries decline: a review of reviews. European J Oral Sci. 1996 Aug;104(4):436-43.
34. Petrou I, Heu R, Stranick M, Lavender S, Zaidel L, Cummins D et al. A breakthrough therapy for dentin hypersensitivity: how dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teeth. J Clin Dent. 2009;20(1):23-31.
35. Rees JS, Addy M. A cross-sectional study of dentine hypersensitivity. J Clin Periodontol. 2002 Nov;29(11):997-1003.
36. Ritter AV, de L Dias W, Miguez P, Caplan DJ, Swift Jr EJ. Treating cervical dentin hypersensitivity with fluoride varnish: a randomized clinical study. J Am Dent Assoc. 2006 Jul;137(7):1013-20; quiz 29.
37. Rohanizadeh R, LeGeros RZ, Fan D, Jean A, Daculsi G. Ultrastructural properties of laserirradiated and heat-treated dentin. J Dent Res. 1999 Dec;78(12):1829-35.
38. Schmalz G, Hiller KA, Nunez LJ, Stoll J, Weis K. Permeability characteristics of bovine and human dentin under different pretreatment conditions. J Endod. 2001 Jan;27(1):23-30.
39. Stabholz A, Khayat A, Weeks DA, Neev J, Torabinejad M. Scanning electron microscopic study of the apical dentine surfaces lased with Nd: YAG laser following apicectomy and retrofill. Int Endod J. 1992 Nov;25(6):288-91.
40. Swift Jr EJ, Perdigao J, Heymann HO. Bonding to enamel and dentin: a brief history and state of the art, 1995. Quintessence Int. 1995 Feb;26(2):95-110.
41. Umberto R, Claudia R, Gaspare P, Gianluca T, Alessandro del V. Treatment of dentine hypersensitivity by diode laser: a clinical study. Int J Dent. 2012;2012:858950.
42. Wara-aswapati N, Krongnawakul D, Jiraviboon D, Adulyanon S, Karimbux N, Pitiphat W. The effect of a new toothpaste containing potassium nitrate and triclosan on gingival health, plaque formation and dentine hypersensitivity. J Clin Periodontol. 2005 Jan;32(1):53-8.
43. West NX, Hughes JA, Addy M. The effect of pH on the erosion of dentine and enamel by dietary acids in vitro. J Oral Rehabil. 2001 Sep;28(9):860-4.
44. Yonaga K, Kimura Y, Matsumoto K. Treatment of cervical dentin hypersensitivity by various methods using pulsed Nd:YAG laser. J Clin Laser Med Surg. 1999 Oct;17(5):205-10.
 Corresponding author:
Corresponding author:
Fabio Antonio Piola Rizzante
Departamento de Dentística – Faculdade de Odontologia de Bauru – Universidade de São Paulo
Al. Dr. Octávio Pinheiro Brisolla, 9-75 – Vila Universitária
CEP 17012-901 – Bauru – SP – Brasil
E-mail: fabio.rizzante@usp.br
Received for publication: December 17, 2013
Accepted for publication: February 26, 2014













