Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.3 Joinville Jul./Set. 2014
ORIGINAL RESEARCH ARTICLE
Analysis of active chlorine releasing and pH of sodium hypochlorite solutions used in Endodontics
Cristhiany Martins Lopes Machado I; Antônio Henrique Braitt I; Gladyvam Rabêlo Braitt I; Evaldo Almeida Rodrigues I; Carlos Eduardo da Silveira Bueno II
I Specialization Course in Endodontics, Health Science Institute, Funorte/Soebras, Núcleo Ilhéus – Ilhéus – BA – Brazil
II São Leopoldo Mandic Post-graduation Center – Campinas – SP – Brazil
ABSTRACT
Introduction: The use of a suitable irrigation solution and a correct root system shape effectively contributes to the success of endodontic treatment. Among the irrigating solutions used in Endodontics, sodium hypochlorite has been the most used because of many qualities. However, this substance must have chemical stability of its properties, by maintaining the potential of hydrogen (pH) and chlorine concentrations appropriately. Objective: The aim of this study was to evaluate active chlorine releasing and pH of some sodium hypochlorite solutions used in endodontic clinical practice. Material and methods: The solutions tested in this study were 0.5% Dakin, 1% Milton, 2% Chlorinated Soda, and household bleaches (Brilux® and Qboa®), which were opened at the same period and first used, with the same storage modus but with different manufacturing dates. The pH was measured with a digital device, and the active chlorine content was obtained through iodometric titration. Results: All solutions presented chlorine content not smaller than that informed in the flasks, ranging from 1% to 2.4%; pH was higher in all solutions, between 9 and 13. Conclusion: Based on the method applied and the results obtained, it was possible to conclude that sodium hypochlorite solutions used specifically in Dentistry (Dakin and Milton solutions and Chlorinated Soda), showed on the labels the hypochlorite content. On the other hand, household bleaches (Brilux® and Qboa®) showed the chlorine content. It was not possible to compare pH authenticity, due to lack of description on the label.
Keywords: Endodontics; sodium hypochlorite; pH; irrigation solutions.
Introduction
Endodontic treatment success is directly related to the comprehensive respect to its different phases: biomechanical preparation, disinfection and root canal filling 12.
Sodium hypochlorite solution has been employed as adjuvant chemical substance in chemo-mechanical preparation of root canals worldwide 10.
The excellent properties of this irrigant accounts for its good acceptance, such as: dissolution of organic tissues, antimicrobial, alkaline pH, bleaching, deodorant, and low surface tension 13.
Siqueira Jr. et al. 20 also affirmed that sodium hypochlorite is the solution most used for chemo-mechanical preparation mainly because its activity of organic matter dissolution.
Estrela et al. 9 and Ludwing et al. 11 cited some of the most important chemical properties of sodium hypochlorite: antimicrobial action, capacity of dissolving organic tissue and saponification. Such properties account for the greatest acceptance as irrigant. Many solutions are available with this same goal, such as EDTA and Endo-PTC. Some studies have demonstrated that such solutions are associated with sodium hypochlorite to obtain better results of antimicrobial action by improving the chemical properties of sodium hypochlorite 1,5,6,8,14,15,17-19.
Pécora and Estrela 16 justifies that the interaction between the physicochemical factors (antimicrobial action of an adjuvant irrigant solution) and the mechanical factors involved in the root canal shaping intensifies the sanitizing process, making their use indispensable.
Sodium hypochlorite at low concentrations can be ineffective against some microorganisms. For example, the best results against Enterococcus faecalis were obtained at 5.25% concentration in comparison with lower concentrations 2.
If some sodium hypochlorite properties are altered, such as the active chlorine content and hydrogen potential (pH), the abnormal parameters may contribute for endodontic treatment failures considering that proper solution storage avoids the chemical variations 3.
Root canal shaping is faster because of the use of new technologies, such as rotary instrumentation. Thus, the use of irrigants of stronger action (sodium hypochlorite at higher concentration) is justified because of the shorter chair time 15.
Among all endodont ic irrigants, sodium hypochlorite is the most used because of its powerful antimicrobial action, capacity of dissolving organic matter, lubricant, and low cost 17.
The active chlorine content may vary according to the irrigant use necessity. No consensus on the ideal sodium hypochlorite concentration in Endodontics has been achieved because although high concentrations account for periapical tissue irritation, they are required against larger bacterial infiltrate (antimicrobial action); while low concentrations show better biocompatibility consequently with low periapical tissue irritation and effectiveness against lower bacterial infiltrate 5.
Aiming to analyze whether the sodium hypochlorite solutions used in clinical practice have desirable quality for Endodontics, studies have been conducted to assess pH alkalinity and stability throughout the usage period, which is an indispensable property to maintain the stability of sodium hypochlorite solutions.
Thus, it is necessary to evaluate the active chlorine content and pH of sodium hypochlorite solutions most used in endodontic clinics aiming to obtain the best performance of these irrigants within root canal system.
Material and methods
Preparation of solutions
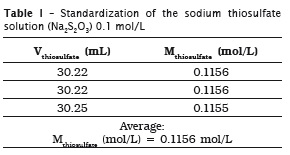
Because potassium iodate is a primary standard, it was used to standardize sodium thiosulfate solution. For this purpose, a mass of 12461 g of potassium iodate was measured, diluted into distilled water, and this mixture was transferred into a volumetric flask of 250 mL, completed up to the measurement mark.
Sodium thiosulfate solution was prepared by weighing approximately 24.8 g of sodium thiosulfate to achieve one liter of the solution, whose desirable concentration was of 1.0 mol/L.
All procedures were made in triplicate, which is the minimum acceptable for showing an analytical result.
Procedure steps
Standardization of sodium thiosulfate solution (Na2S2O3) 0.1 mol/L
Inside an Erlenmeyer flask, an aliquot of 25 mL of potassium iodate (KIO3) 0.02 mol/L was added to 10 mL of 10% potassium iodide (KI) m/v. Following, this mixture was acidified with 3 mL of sulfuric acid (H2SO4), moment at which the solution showed a brown color. The mixture was titrated with sodium thiosulfate solution up to reach a pale yellow color. Next, 3 mL of 3% m/v starch solution, with an intense blue color, was added; titration was continued up to obtain a colorless solution. The volumes were recorded and the concentration of the sodium thiosulfate was determined. This method was based on the procedures described by Vogel 22.
IO3 - + 5I- + 6H+ ⇔ 3I2 + 2H2O (eq. 1)
The acid stoichiometry is the same of the acid.
6 Miodate x Viodate = Mthiosulfate x Vthiosulfate in which:
Miodate: potassium iodate molarity, which corresponding to 0.02329 mol/L;
Viodate: potassium iodate volume added to the mixture, which corresponding to 25 mL;
Mthiosulfate: thiosulfate concentration that is being determined, in mol/L;
Vthiosulfate: sodium thiosulfate volume used in titration.
The addition of excessive potassium iodide was necessary to avoid iodine (I2) volatilization formed during titration process. The addition of sulfuric acid was executed to increase the potential of reduction of iodine-iodide system, and the iodine amount released during the titration process was equivalent to the solution acidity.
The reaction of chlorine with sodium hydroxide base is necessary to obtain sodium hypochlorite, according to equation no. 2.
2 NaOH + Cl2 NaOCl + NaCl + H2O (eq. 2)
According to the stoichiometry, it was possible to deduce that:
Cl2 = 71 NaClO 74,5 (eq. 3)
Cl2 = 0,953 NaClO (eq. 4)
Meaning that:
% sodium hypochlorite x 0.953 = % active chlorine (eq. 5)
The determination of the sodium hypochlorite content in the commercial solutions was based on the procedures described by Vogel [22], with modifications.
Procedure
Inside a 250 mL Erlenmeyer f lask, it was added 5 mL of the sample, 25 mL of distilled water, 2 mL of 10% m/v potassium iodide and 3 mL of glacial acetic acid. This mixture was titrated with standardized sodium thiosulfate up to obtain a pale yellow color. Next, 3 mL of 3% m/v starch solution showing an intense blue color was added; continuing the titration up to reach a colorless mixture. The volumes were recorded and the sodium hypochlorite concentrations of the commercial solutions were determined.
Inside the Erlenmeyer f lask containing the samples, the following reactions occurred:
OCl- + 2 I- + 2H+ ⇔ Cl- + I2 + 2H2O (eq. 6)
I2 + S2O3 2- ⇔ 2I- + S4O6 2- (eq. 7)
Meaning that:
2 mhypochlorite = Mthiosulfate x Vthiosulfate MMhypochlorite (eq. 8)
in which:
Mhypochlorite: sodium hypochlorite mass in the aliquot (g);
MMhypochlorite: sodium hypochlorite molar mass, which corresponding to 74.5 g;
Mhtiosulfate: sodium thiosulfate molarity, which corresponding to 0.1156 mol/L;
Vthiosulfate: thiosulfate volume used to titrate each sample (L).
After finding the sodium hypochlorite mass corresponding to 5 mL in the sample aliquot, a rule of three was carried out to determine the sodium hypochlorite concentration (percentage). Following, this value was replaced in equation no. 5 and the active chlorine content was determined.
Mhypochlorite ........ 5
x ........ 100
x ⇒ correspond to the hypochlorite content in the sample.
Results
All analyses were made in triplicate. In samples #1, #2 and #3 the labels exhibited the hypochlorite content, while in samples #4 and #5 the labels did not show the chlorine content. Accordingly, the following tables present the sample data in which either the sodium hypochlorite or chlorine concentrations were determined. When the chlorine content was specified on the label, this information is shown in the tables.

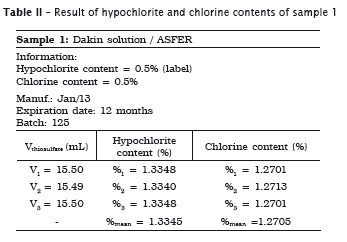
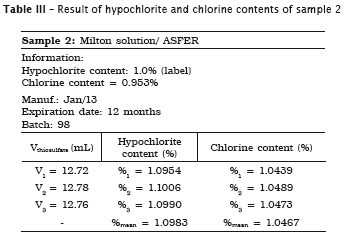

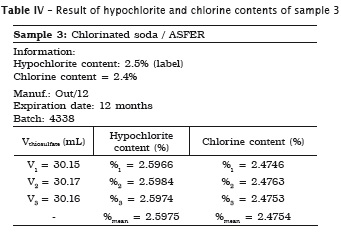

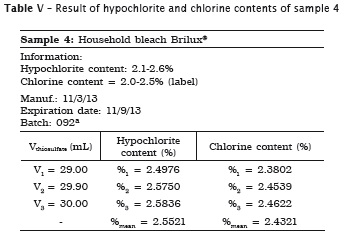

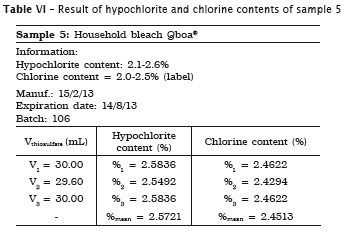

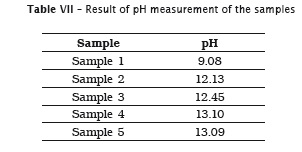
Also, pH of the samples was measured, but because of lack of label information by the manufacturer, it was not possible to verify its authenticity. Table 7 provides the data.
Discussion
Among the main requirements of endodontic treatment success, the complete elimination of all debris from root canal system during shaping is mandatory. The mechanical action performed by the instruments occurs only in the main canal lumen, not reaching the root canal system. Considering all this morphological complexity, the irrigant is chemically essential. According to Borin et al. 3, the irrigant action promoted the hydraulic circulation within root canal, acting on the organic matter and promoting tissue dissolution. Accordingly, the irrigant removes the dentine debris from the instrument cut, making possible the disinfection and cleaning of all root canal system.
Facing all endodontic irrigants available in Brazilian dental market, the dentist should choose a solution presenting satisfactory properties to obtain endodontic treatment success. It is important that the irrigant features continues unaltered during all storage time, so that caution is required to maintain the stability of each substance 6.
Sodium hypochlorite is found in Brazilian dental stores, compounding pharmacies, and even supermarkets. In some cases, the dentist only has access to the latter, and uses household bleach to replace easily a dental sodium hypochlorite. Thus, this study aimed to verify the chlorine content existing in solutions found in supermarkets.
The Brazilian wa rm weather and the characteristics of distribution and storage of dental products in the commercial places may result in problems during the use of sodium hypochlorite with active chlorine content smaller than that required. This occurs because of the instability of the solutions, which can be aggravated by warm, storage time, and even the use of inappropriate flasks.
Clarkson et al. 7 reinforced the need of storing sodium hypochlorite inside closed opaque flasks because the constant opening of these flasks may cause loss of chlorine concentration diluted within the solutions, leading to faster pH decrease.
The manufacturing negligence (use of improper water, inadequate storage) may also lead to the decrease of free chlorine content, resulting in the early decomposition of the product, and consequently, in the loss of antimicrobial power 21.
The iodometry was the method employed to analyze chemically and verify the active chlorine content, because it has been very used in literature. Iodometry may be very used because it is a totally manual and colorimetric technique, in which many dilutions are required to reach the titration itself. However, it is a sensible technique, and during the execution, a drop added or subtracted can result in difference in the final result.
Conclusion
Considering the methodology and the obtained results, it can be concluded that:
1. the sodium hypochlorite solutions specifically used in Dentistry (Dakin and Milton solutions and chlorinated soda) exhibited the hypochlorite content on the label, while the household bleaches (Brilux® and Qboa®) exhibited the chlorine content information on the label;
2. Milton solution and chlorinated soda showed the hypochlorite content similar to that specified on the label;
3. the household bleaches (Brilux® and Qboa®) showed the chlorine content similar to that specified on the label;
4. Dakin solution was the only irrigant showing a significant increase of hypochlorite content of 1.33%;
5. all solutions showed a high pH from 9.08 to 13.09; however, it was not possible to compare pH authenticity due to lack of information on the label.
References
1. Beltz RE, Torabinejad M, Pouremail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentine. J Endod. 2003;29(5):334-7. [ Links ]
2. Berber VB, Gomes BPFA, Sena NT, Vianna ME, Ferraz CCR, Zaia AA et al. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. I Endod J. 2006;39:10-7.
3. Borin G, Becker AN, Oliveira EPM. A história do hipoclorito de sódio e a sua importância como substância auxiliar no preparo químico mecânico de canais radiculares. Rev Endod Pesq Ens Online. 2007;3(5).
4. Borin G, Oliveira EPM. Alterações no pH e teor de cloro ativo em função da embalagem e local de armazenamento de solução de hipoclorito de sódio em diferentes concentrações. RFO UPF. 2008;13(2):45-50.
5. Câmara AC, Albuquerque MM, Aguiar CM. Soluções irrigadoras utilizadas para o preparo biomecânico de canais radiculares. Pesq Bras Odontopediatria Clín Integr. 2010 Jan/ Apr;10(1):127-33.
6. Camargo SEA, Blanco TM, Lima RY, Rode SM, Camargo CHR. Avaliação do pH das soluções de hipoclorito de sódio 1% e 2,5% e digluconato de clorexidina 2% em função do tempo. Rev Odonto. 2008;16(31):85-91.
7. Clarkson RM, Mouler AJ, Podlich HM. The shelflife of sodium hypochlorite irrigating solutions. Aust Dent J. 2001;46(4):269-76.
8. Ercan E, Ozekinci T, Atakul F, Gul K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. J Endod. 2004;30(2):84-7.
9. Estrela C, Estrela CRA, Barbin EL, Spanó JCE, Marchesan MA, Pécora JD. Mecanismo de ação do hipoclorito de sódio. Braz Dent J. 2002;13(2):113-7.
10. Johnson BR, Remeikis NA. Effective shelflife of prepared sodium hypochlorite solutions. J Endod. 1993;19(1):40-3.
11. Ludwig A, Hoffmeister MK, Irala LED, Salles AA, Limongi O, Soares RG. Análise da concentração de cloro ativo e pH em amostras de hipoclorito de sódio 1%. RSBO. 2007;4(1):29-35.
12. Machtou P. Irrigation in endodontics. Actual Odonto Stomotol. 1980;34(131):387-94.
13. Marchesan MA, Souza RA, Guerisoli DMZ, Silva RS, Pécora JD. Análise de algumas propriedades físico-químicas das águas sanitárias encontradas no mercado brasi leiro. RBO. 1998;55(5):301-3.
14. McGurkin-Smith R, Trope M, Caplan D, Sigurdsson A. Reduction of intracanal bacteria using GT rotary inst rumentat ion, 5.25% NaOCl, EDTA, and Ca(OH)2. J Endod. 2005; 31(5):359-63.
15. Monteiro FG, Bombana A, Santos M, Zaragoza RA. Análise da limpeza dentinária em canais radiculares preparados com um sistema rotatório e diferentes substâncias químicas. RGO. 2008;56(1):7-15.
16. Pecora JD, Estrela C. Hipoclorito de sódio. In: Estrela C (Org.). Ciência endodôntica. 1. ed. São Paulo: Artes Médicas; 2004.
17. Ribeiro ECC. Estabilidade química das soluções de hipoclorito de sódio [dissertação de mestrado]. São Paulo: Universidade de São Paulo. Faculdade de Odontologia, 2009.
18. Sassone LM, Fidel RAS, Murad CF, Fidel SR, Hirata Jr R. Antimicrobial activity of sodium hypochlorite and chlorhexidine by two different tests. Aust Endod J. 2008;34:19-24.
19. Sena NT, Gomes BPFA, Vianna ME, Berber VB, Zaia AA, Ferraz CCR et al. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. I Endod J. 2006;39:878-85.
20. Siqueira Jr JF, Rôças IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5% and 5.25% sodium hypochlorite. Int End J. 2000;26:331-4.
21. Ventura ACA, Sestari V, Collesi RR, Sampaio JMP. Determinação do teor de cloro ativo nas soluções de hipoclorito de sódio: visão atual do problema. Rev Paul Odontol. 2002;4:24-8.
22. Vogel AI. Análise química quantitativa. 6. ed. Rio de Janeiro: LTC; 2002.
 Corresponding author:
Corresponding author:
Antônio Henrique Braitt
Av. Aziz Maron, 1.117/703
CEP 45605-904 – Itabuna – BA
E-mail: henrique_braitt@terra.com.br
Received for publication: January 20, 2014
Accepted for publication: February 27, 2014













