Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.3 Joinville Jul./Set. 2014
Literature Review Article
Clinical aspects and pharmacological treatment of trigeminal neuralgia
Renata Cristiane dos Reis I; Carina Fernanda Mattedi Nones I; Caroline Machado Kopruszinski I; Wagner Hummig I; Juliana Geremias Chichorro I
I Department of Pharmacology, Federal University of Paraná – Curitiba – PR – Brazil
ABSTRACT
Introduction: Trigeminal neuralgia (TN) is defined as sudden, usually unilateral, severe and brief pain episodes within the distribution of one or more branches of the trigeminal nerve. In some patients a constant background pain may persist, additionally to pain attacks, which can make difficult to differentiate the trigeminal neuralgia from other orofacial pain types. Objective: To review the classification, physiopathological aspects, epidemiologic data and pharmacological options to control pain related to trigeminal neuralgia. Literature review: One of the proposed etiologies for this condition is a localcircumscribed demyelination of the trigeminal nerve resulting in neuronal hyperexcitability and generation of ephaptic coupling, which would be responsible for the pain paroxysms. Initially, the treatment of patients with these pain characteristics is based on the use of anticonvulsants, in order to attenuate the ectopic-generated pain impulses. Carbamazepine is the first-line drug, but other anticonvulsants may be employed and have shown variable efficacy in the treatment of trigeminal neuralgia. Conclusion: According to the new classification of the International Headache Society, classic trigeminal neuralgia is divided in purely paroxysmal and with concomitant persistent facial pain. The pathophysiology is unclear, but trigeminal neuralgia seems to be the consequence of vascular compression of the trigeminal nerve near the brain stem. Although TN presents a low prevalence in general population (i.e. 5-30 new patients per 100,000), trigeminal neuralgia is an important clinical concern both by pain severity and difficulty of its satisfactory control. Anticonvulsants are the medication of choice in the treatment of trigeminal neuralgia; however, their use is associated with several adverse effects and possibility of treatment refractoriness.
Keywords: trigeminal neuralgia; chronic pain; anticonvulsants.
Introduction
Neuropathic pain is defined as the pain resulting from a lesion or dysfunction of somatosensory system and characterized by either spontaneous pain or sensitivity exacerbated to different stimuli 22. The most common type of craniofacial neuropathic pain is trigeminal neuralgia (TN), whose prevalence is estimated in 5-30 individuals at every 100,000 43.
Trigeminal neuralgia may occur at any age; however, in 90% of the cases affects people aged more than 40 years 14, with greater incidence in females (2:1 ratio) 14,26,32.
This disorder has been described in the literature for centuries. References to unilateral facial pain responsible for facial spasms can be found in the writings of Aretaeus and Jujani, at 2nd and 11th centuries, respectively. In 1773, Fothergill described the typical features of trigeminal neuralgia, including its paroxysmal natural and association with triggering factors 13.
Trigeminal neuralgia is def ined by the International Association for the Study of Pain (IASP) as a "sudden, usually unilateral, severe and brief pain episodes occurring in one the distribution of one or more branches of the trigeminal nerve" 32,46 and characterized by severe, acute, electric shock-like piercing pain, followed by refractory period 3,24. Pain episodes are normally triggered by stimulation of specific areas, so-called trigger points or zones, localized in the area innervated by trigeminal nerve 26. In most trigeminal neuralgia cases, only one branch is affected, mainly the maxillary, and about 30% of cases involve maxillary and mandibular branches. Both ophthalmic branch involvement and bilateral trigeminal neuralgia cases are rare 24. Triggering stimuli of pain attacks include the acts of speaking, toothbrushing, shaving, drinking, soft touching on face, among other 14,42. The patients suffering trigeminal neuralgia have marked reduction in quality of life because they avoid any routine task that can trigger a pain crisis 24.
According to the new classification of the International Headache Society (IHS) 18, classic trigeminal neuralgia is caused by neurovascular compression more frequently through the superior cerebellar artery and is divided into classical trigeminal neuralgia purely paroxysmal and classical trigeminal neuralgia with concomitant facial persistent facial pain. Before this new classification, trigeminal neuralgia was also classified as symptomatic and included cases in which the neuralgia was associated with other disorders such as traumas, tumors and multiple sclerosis. Regardless of the etiology, neuropathic pain affecting trigeminal nerve reaches high rates in pain scales, which raises further interest in its study 17.
The treatment of choice of trigeminal neuralgia is pharmacological. Firstly, many patients have been beneficiated with anticonvulsants, such as carbamazepine, gabapentin and pregabalin. Notwithstanding, many patients do not respond to or become refractory to pharmacological treatment. Also, some patients have pain reduction together with side effects intolerable enough to justified pharmacological treatment suspension. The aforementioned cases require multimodal management, associating drugs with surgery 1. Nevertheless, neither pharmacological nor surgical treatment for trigeminal neuralgia provides considerable pain relief in all patients, justifying the need of further studies to contribute in understanding the physiopathology of this disorder 7.
The aim of this study was to review the classification, physiopathological, epidemiological data and the pharmacological options for pain control associated with trigeminal neuralgia.
Literature review
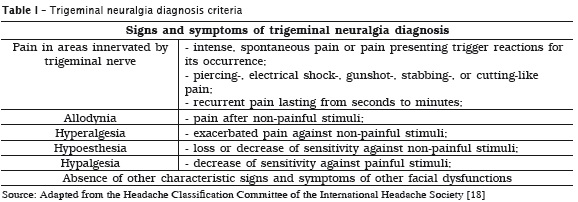
Although trigeminal neuralgia (TN) is not a new disease, specific examinations and tests aiding in diagnosis does not exist 6,12,40. Notwithstanding, many signs and symptoms characterized TN and their evaluation is the main tool for diagnosing this neuropathic pain type. TN main diagnosis criteria are severe, recurrent, paroxysmal, electrical shock-like pain affecting only the areas innervated by trigeminal nerve, lasting from seconds to minutes, together with other signs and symptoms described in table 1 2,4,6,12,18,25,39,40,45,47. Because TN is a rare pain type, many health professionals do not recognize it, making difficult the correct diagnosis. Accordingly, some studies have shown that TN diagnosis may take more than one year in patients suffering of facial pains 6,40.
As aforementioned cited, recently a new TN classification has been proposed by IHS, in which TN is divided into classical trigeminal neuralgia purely paroxysmal and classical trigeminal neuralgia with persistent concomitant pain. The diagnosis of classical TN purely paroxysmal, the most common type, should comprise at least three signs and symptoms described in table 1, including recurrent facial pain attacks, without persistent facial pain [18]. To diagnosis classical TN with persistent concomitant pain, one may detect at least three signs and symptoms presented in table 1, including recurrent facial pain attacks, with persistent facial pain of moderate to severe intensity 4,18.
To make easy and improve not only TN diagnosis, but also the diagnosis of many other diseases of di ff icult diagnosis, quant itat ive sensory test (QST) has been applied, composed of the sensory stimulation of the affected area and observation of the patients' reactions against stimulation 20,45,48. Currently, a set of sensory tests comprising 13 thermal and mechanical parameters (i.e., evaluation of pain threshold against different temperatures and forces) was standardized also for sensory facial alterations, such as trigeminal neuralgia 45. This test enables evaluating the particular features of each TN patient, thus contributing for a better treatment and better control of sensory alterations in orofacial area 20,45,48.
Many studies have been developed aiming to clarify the physiopathological mechanisms involved in TN development and the correlation with some diseases' characteristics, i.e. paroxysmal attacks generally triggered by innocuous stimulus 18.
Since the observation of the correlation between the vascular compression of trigeminal nerve at brainstem area with TN development 8,21, studies have aimed to confirm this correlation and identify the factors responsible for triggering the neuralgia associated with this alteration, which has been currently considered as TN main cause 18.
Accordingly, a study analyzed 41 TN clinical cases and observed the vessel contact with the nerve in 37 patients (90%); and of these, in 25 cases (68%) the contact was at the entering area of trigeminal nerve in brainstem. The compression origin was exclusively venous in 25% of the cases; exclusively arterial in 76%; and mixed in 13%. Of 33 patients submitted to vascular decompression, 23 (70%) reported significant pain relief. Control group comprised cadaver dissections after perfusion at physiologic pressure levels, and vascular contact with trigeminal nerve occurred in 40% of the cases, demonstrating that the vascular compression of trigeminal nerve is an anatomic abnormality with high correlation with TN 16.
Since vascular compression importance was established in TN, many advances have been obtained in the Discovery of mechanisms associated with the development of this clinical condition.
Many researches have been proposed that TN is probably consequence of specific abnormalities of trigeminal afferent neurons at trigeminal root area 11,47. Part of these abnormalities include structural alteration in the root affected by compression such as local-circumscribed demyelination and juxtaposition of demyelinated axons 19,28; presence of few thin-myelinated axons adjacent to demyelination zone, which may ref lect both in demyelination and remyelination or partial demyelination of affected fibers; cases in which a single thin myelin sheath involving many adjacent axons suggesting the occurrence of aberrant remyelination 27,28; presence of great number of collagen fibers in extracellular matrix 34,28, alterations in oligodendrocytes 30, among others.
In addition to both demyelination and lesions in axons and in central myelin found in vascular compression site, the literature has described abnormalities in peripheral myelin at the area close to the lesion, which were more pronounced in longest TN patients. Of these, a little number of peripheral axons was demyelinated; however, many axons were atrophic, hypertrophic, contracted or swollen, and the myelin sheaths that varied in shape and thickness 30.
Recently alterations in central nervous system of TN patients have been described by presenting significant reduction in the grey matter volume of many structures associated with pain processing and perception, such as thalamus and somatosensory cortex, among others 31. Also, it has been found a correlation of the disease duration with the volume reduction of grey matter at anterior cingulate cortex, a structure that is likely involved in developing chronic pains 29,31. These alterations complement that aforementioned cited would justify partly the persistent facial pain reported by some TN patients.
Attempting to correlate the paroxysmal pain attacks with structural alterations observed in afferent sensory trigeminal neurons, Devor et al. (2002) 10 formulation the ignition hypothesis, which has been the most largely accepted to explain TN physiopathology. This hypothesis states that sensory neurons partially damaged become hyperexcitable and susceptible to cross excitation, which coming from the physical proximity of the neurons to the site of root compression. Therefore, the explosions of post-trigger neuronal activity recruit additional neighboring neurons leading to a rapid accumulation of electrical activity, which can be amplified by ephaptic interaction among neurons, since myelin sheath was damaged and nervous fibers maintain close contact among them. Thus, the stimulus of a single sensory fiber may lead to activate many others, and the explosions of neuronal activity triggered by an external stimulus many extended beyond the stimulus duration. According to this hypothesis, pain paroxysms experienced by TN patients would be the result of this synchronized phenomenon, which would be stopped by hyperpolarization coming from potassium ion influx making the neurons refractory to new excitation and would partly justify the refractory period after the crisis 10,11,33,47.
Ignition hypothesis can explain part of the mechanisms involved in one of the main TN characteristics: paroxysmal pain attacks. Notwithstanding, other physiopathological process may contribute for the appearance of these and other symptoms, such as hyperalgesia and allodynia reported by some patients; and persistent facial pain, which is characteristic of one classical TN types.
Accordingly, some studies have suggested that alterations in voltage-gated sodium channels (Nav) may play an important role in TN physiopathology. Maybe the expression of sodium channels increased in demyelinated areas, which account for the greater neuronal excitability and ectopic shooting of afferent trigeminal fibers. This increased expression of sodium channels detected in gingival tissue samples of TN patients, which exhibited greater expression of Nav1.3 sodium channels than that of control group. Considering that sodium channels blocker drugs, as carbamazepine, have been normally effective in TN pain control, it is possible that sodium channels play a relevant role in TN physiopathology 37.
Many studies have provided advances in TN development. However, it is still necessary developing methodologies that allow elucidating TN physiopathological mechanisms to be correlated with TN extremely damaging clinical aspects, so that new treatment strategies can be developed.
Currently, the initial treatment of TN patients is pharmacological and based on anticonvulsants, and carbamazepine is the drug of choice. Surgical treatment can also be considered either when patients' symptoms do not improve, or patients cannot tolerate pharmacological treatment, or even in relapse cases. Surgical treatments are not the issue of this literature review, but a detailed description and indications, risks and benefits of each surgical procedure has also been presented and discussed by other authors 9,24,25,39.
Carbamazepine has been used in TN pain control since the 1960s, and studies have been indicated that carbamazepine is effective in reducing TN symptoms in up to 80% of the patients 5,35,36. Mainly because the high effectiveness in TN treatment, carbamazepine has also been used for differential diagnosis between TN and other orofacial pains. Accordingly, it has been estimated that carbamazepine's number needed to treat (NNT) in TN is equal to 2.5, that is, at each 2.5 patients treated with carbamazepine, 1 is benefited from treatment 44.
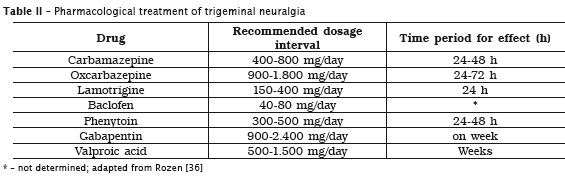
Despite of the high effectiveness of carbamazepine in TN pain control, long-term treatment has been associated with many side effects including: sleepiness, dizziness, nausea, vomiting, ataxia, renal and hepatic toxicity, and symptoms relapse in up to 50% of the patients 9. According to Wiffen et al. (2005) 44, 3.7 is the NNT required to cause adverse side effects with carbamazepine use. Moreover, carbamazepine is a potent inducer of its own metabolism, thus, the therapeutic level is not maintained whether the dose is not adjusted. It has been suggested to increase carbamazepine dosage approximately 20 days after the beginning of treatment. Carbamazepine half-life time is altered by longer use, ranging from 20-40 hours to 11-27 hours. These pharmacokinetic characteristics justify carbamazepine effectiveness loss whether dosage adjustments are not performed. Additionally, it is recommended to start treatment with low dosage (100 mg/day), which should be increased to 100-200 mg at every three days until patient reports pain relief. Maintenance dosage is generally of 400 to 800 mg/day (at every 12 hours), but some patients can need up to 2,400 mg/day, at every 6 hours 36,39. Taking into consideration both side effects and pharmacokinetic features of carbamazepine, the effectiveness of other drugs has been evaluated for TN treatment. Accordingly, it has been suggested that the anticonvulsant oxcarbazepine is also effective in controlling pain despite of the small evidences on its effectiveness, and longer oxcarbazepine use is related to smaller side effects than that of carbamazepine 15. In case of intolerance (approximately 5 to 19% of patients are intolerant to carbamazepine) or lack of benefit with first- and second-choice drugs (carbamazepine and oxcarbazepine, respectively), the use of lamotrigine, baclofen, phenytoin, valproic acid and gabapentin can be considered 15,25,36. Table 2 displays the pharmacological options to treat TN, the dosages recommended, and the time for pain relief of each drug 36.
The main mechanisms of anticonvulsant analgesic effects in neuropathic pains are blockage of sodium and/or calcium channels, increase of GABA inhibitory neurotransmission, reduction of GABA inhibitory neurotransmission 41. Neuropathic pains result from the lesion of sensory neurons leading to alterations, among which the change in sodium/calcium channel expression changes. These alterations seemed to be associated with spontaneous pain development, hyperalgesia and allodynia, common symptoms in neuropathic pain cases. Many anticonvulsants act inhibiting both sodium and calcium channels, thus reducing GABA neurotransmission. This mechanism has been proposed by carbamazepine, oxcarbazepine and lamotrigine 23. However, some differences have been observed in the mechanism of action of each one of these drugs, i.e. oxcarbazepine inhibits many voltage-gated sodium channels, which are not affected by carbamazepine 38. These differences in the mechanism of action could explain the difference in anticonvulsant drug effectiveness in TN treatment. Finally, according to new IHS classification, while classical TN purely paroxysmal firstly responds well to pharmacological treatment with either carbamazepine or oxcarbazepine, classical TN with persistent facial pain does not respond well to both conservative and neurosurgical managements 18.
Conclusion
According to the new classification of the International Headache Society, classical trigeminal neuralgia is divided into purely paroxysmal and with concomitant persistent facial pain. Trigeminal neuralgia physiopathology is not fully clarified, but it seems to be the consequence of the vascular compression of trigeminal nerve at the entry of the roots in the brainstem. Despite of the small trigeminal neuralgia prevalence in population (5-30 individuals at every 100,000), it is very clinically relevant because of both pain severity and difficulty in satisfactory pain control. Anticonvulsants are the drug of choice for trigeminal neuralgia; however, longer use has been associated with many side effects and possible treatment refractoriness.
References
1. Arai YP, Hatakeyama N, Nishihara M, Ikeuchi M, Kurisuno M, Ikemoto T. Intravenous lidocaine and magnesium for management of intractable trigeminal neuralgia: a case series of nine patients. J Anesth. 2013;27(6):960-2. [ Links ]
2. Bennetto L, Patel NK, Fuller G. Trigeminal neuralgia and its management. BMJ. 2007;334(7586):201-5.
3. Benoliel R, Sharav Y. Chronic orofacial pain. Curr Pain Headache Rep. 2001;14(1):33-40.
4. Benoliel R, Birman N, Eliav E, Sharav Y. The international classification of headache disorders: accurate diagnosis of orofacial pain? Cephalalgia. 2008;28(7):752-62.
5. Blom S. Trigeminal neuralgia: its treatment with a new anticonvulsant drug (G-32883). Lancet. 1962;1(7234):839-40.
6. Boto GR. Trigeminal neuralgia. Neurocirugia (Astur). 2010;21(5):361-72.
7. Chichorro JG, Zampronio AR, Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain. 2006;123(1-2):64-74.
8. Dandy WE. Concerning the cause of trigeminal neuralgia. Am J Surg. 1934;24:447-55.
9. Delzell Jr JE, Grelle AR. Trigeminal neuralgia. New treatment options for a well-known cause of facial pain. Arch Fam Med. 1999;8(3):264-8.
10. Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18(1):4-13.
11. Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg. 2002;96(3):532-43.
12. Edlich RF, Winters KL, Britt L, Long 3rd WB. Trigeminal neuralgia. J Long Term Eff Med Implants. 2006;16(2):185-92.
13. Eller JL, Ahmed MR, Burchiel KJ. Trigeminal neuralgia: definition and classification. Neurosurg Focus. 2005;18(5).
14. El-Tallawy HN, Farghalya WM, Rageha TA, Shehatta GA, Hakeem NA, Badry R et al. Prevalence of trigeminal neuralgia in Al-Quseir city (Red sea Governorate), Egypt. Clin Neuroland Neurosurg. 2013;1-3.
15. Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71(15):1183-90.
16. Hamlyn PJ, King TT. Neurovascular compression in trigeminal neuralgia: a clinical and anatomical study. J Neurosurg. 1992;76(6):948-54. 17. Hargreaves KM. Orofacial pain. Pain. 2011;152(3):S25-S32.
18. Headache Classification Committee of the International Headache Society (IHS). The Internacional Classi f icat ion of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
19. Hilton DA, Love S, Gradidge T, Coakham HB. Pathological findings associated with trigeminal neuralgia caused by vascular compression. Neurosurgery. 1994;35(2):299-303.
20. Jääskeläinen SK, Teerijoki-Oksa T, Forssell H. Neurophysiologic and quantitative sensory testing in the diagnosis of trigeminal neuropathy and neuropathic pain. Pain. 2005;117(3):349-57.
21. Janetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1): Suppl:159-62.
22. Jongen JLM, Hans G, Benzon HT, Huygen F, Hartrick CT. Neuropathic pain and pharmacological treatment. Pain Pract. 2013;1-13.
23. Johansen Landmark C. Antiepileptic drugs in non-eplepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
24. Kleef MV, Genderen WEV, Narouze S, Nurmikko TJ, Zundert JV, Geurts JW et al. Trigeminal neuralgia. World Institute of Pain. Pain Pract. 2009;9(4):252-9.
25. Krafft RM. Trigeminal neuralgia. Am Fam Physician. 2008;77(9):1291-6.
26. Liu J, Dai J, Lingling E, Wang D, Liu H. Trigeminal neuralgia may be caused by abnormality of the trigger zone. Med Hypotheses. 2010;74(2010):818-9.
27. Love S, Hilton DA, Coakham HB. Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain Pathol. 1998;8(1):1-11.
28. Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347-60.
29. May A. Structural brain imaging: a window into chronic pain. Neuroscientist. 2011;17(2):209-20.
30. Marinkovic S, Gibo H, Todorovic V, Antic B, Kovacevic D, Milisavljevic M et al. Ultrastructure and immunohistochemistry of the trigeminal peripheral myelinated axons in patients with neuralgia. Clin Neurol Neurosurg. 2009;111(10):795-800.
31. Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. NeuroImage. 2013;74:352-8.
32. Peschillo S, Delfini R. Trigeminal neuralgia: a new neuroimaging perspective. World Neurosurg. 2012;1-3.
33. Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain. 1994;56(2):127-38.
34. Rappaport ZH, Govrin-Lippmann R, Devor M. An electron-microscopic analysis of biopsy samples of the trigeminal root taken during microvascular decompressive surgery. Stereotact Funct Neurosurg. 1997;68(1-4 Pt 1):182-6.
35. Rockliff BW, Davis EH. Controlled sequential trials of carbamazepine in trigeminal neuralgia. Arch Neurol. 1966;15(2):129-36.
36 . Rozen TD. Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin. 2004;22(1):185-206.
37. Siqueira SRDT, Alves B, Malpartida HMG, Teixeira MJ, Siqueira JTT. Abnormal expression of voltage-gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience. 2009;164(2):573-7.
38. Schmidt D, Elger CE. What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav. 2004;5(5):627-35.
39. Scrivani SJ, Mathews ES, Maciewicz RJ. Trigeminal neuralgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(5):527-38.
40. Shakur SF, Bhansali A, Mian AY, Rosseau GL. Neurosurgical treatment of trigeminal neuralgia. Dis Mon. 2011;57(10):570-82.
41. Söderpalm B. Anticonvulsants: aspects of their mechanisms of action. Eur J Pain. 2002;6: SA3-9.
42. Thomas KL, Vilensky JA. The anatomy of vascular compression in trigeminal neuralgia. Clin Anat. 2013;1-5.
43. Wang X, Liang H, Zhou C, Xu M, Xu L. Sensitization induces hypersensitivity in trigeminal nerve. Clin Exp Allergy. 2012;42:1638-42.
44 . Wiffen PJ , McQuay HJ, Moore RA . Carbamazepine for acute and chronic pain. Cochrane Database Syst Rev. 2005;(3):CD005451.
45. Yekta SS, Smeets R, Stein JM, Ellrich J. Assessment of trigeminal nerve functions by quantitative sensory testing in patients and heal thy volunteers. J Oral Maxi llofac Surg. 2010;68(10):2437-51.
46. Zakrzewska JM. Differential diagnosis of facial pain and guidelines for management. British Journal of Anaesthesia. 2013;111(1):95-104.
47. Zakrzewska JM, McMillan R. Trigeminal neuralgia: the diagnosis and management of this excruciating and poorly understood facial pain. Postgrad Med J. 2011;87(1028):410-6.
48. Zaslansky R, Yarnitsky D. Clinical application of quantitative sensory testing (QST). J Neurol Sci. 1998;153(2):215-38.
 Corresponding author:
Corresponding author:
Juliana Geremias Chichorro
Departamento de Farmacologia – Universidade Federal do Paraná
Rua Cel. Francisco Heráclito dos Santos, 210 – Jardim das Américas
CEP 81531-970 – Curitiba – PR – Brasil
E-mail: juliana.chichorro@ufpr.br
Received for publication: August 27, 2013
Accepted for publication: February 3, 2014













