Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.4 Joinville Out./Dez. 2014
ORIGINAL RESEARCH ARTICLE
Analysis of the effect of ultrasonic agitation on the cleaning of root canals using different periods during the final irrigation
Felipe Xavier I; Giselle Nevares I; Diana Santana de Albuquerque I; Luciana Ferraz Gominho II; Rafaela Leal de Alcântara Dellazari I; Rodrigo Sanches Cunha III; Joedy Maria Costa Santa Rosa I
I Department of Operative Dentistry and Endodontics, Dental College of Pernambuco, University of Pernambuco – Camaragibe – PE – Brazil
II Academic Unit of Biologic Science, Federal University of Campina Grande – Campina Grande – PB – Brazil
III Division of Endodontics, University of Manitoba – Winnipeg – MB – Canada
ABSTRACT
Introduction: The ultrasonic agitation was introduced as an adjuvant to conventional chemo-mechanical debridement during endodontic treatment to overcome the persistence of biofilms. Objective: To verify the cleaning of root canals irrigated with sodium hypochlorite (NaOCl) and ethylenediaminetetraacetic acid (EDTA), with or without an ultrasonic agitation, using different time periods and images obtained by scanning electron microscope (SEM). Material and methods: Forty mandibular incisors were cleaned, shaped and randomly divided into five groups according to the final irrigation protocol: SH10 group (ultrasonic agitation with NaOCl for 10 s), SH30 group (ultrasonic agitation with NaOCl for 30 s), SHE30 group (ultrasonic agitation with NaOCl and EDTA for 10 s), SHE90 group (ultrasonic agitation with NaOCl and EDTA for 30 s), and control group (NaOCl and EDTA without ultrasonic agitation). The teeth were prepared and analyzed by SEM at ×2000. The Kruskal–Wallis test was used with a 5% level of significance. Results: For the cervical and medial thirds, there was no statistically significant difference in cleaning among the protocols used (p > 0.05). For the cleaning of the apical third, SHE90 group demonstrated a significant difference (p < 0.05), as compared to the control and SH10 groups. Conclusion: For the final irrigation, an ultrasonic agitation with NaOCl and EDTA for 30 s allowed a better cleaning of the debris in the apical third of the root canal.
Keywords: endodontics; smear layer; sodium hypochlorite.
Introduction
In the presence of infection, one purpose of the mechanical cleaning of the root canal system is the removal of the inner layer of contaminated dentin, by cutting the walls of this anatomical space. However, dentin surfaces may remain untouched after endodontic preparation 32,44, so this disinfection must be complemented by the action of irrigating solutions 10,24. Moreover, during instrumentation, there is the production of dentin shavings, which if left in the root canal alongside the remnants of the necrotic tissues and microorganisms, may lead to the perpetuation of infection and generation of an adverse endodontic treatment prognosis 39.
The need for smear layer removal and the disinfection of the root canal makes irrigation crucial in complementing instrumentation. To this end, a sodium hypochlorite (NaOCl) solution is recommended as the main irrigating solution because of its broad antimicrobial activity and ability to dissolve organic tissue 16. Because of its chelating action, ethylenediaminetetraacetic acid (EDTA) is also recommended as a form of adjuvant irrigation to remove and prevent smear layer formation 45.
Although conventional irrigation (needle/syringe) has been widely used, several techniques and devices have been proposed to optimize the chemical and mechanical properties and improve the penetration of irrigating solutions; these include the use of syringes associated with modified needles, sonic and ultrasonic devices, and negative pressure irrigation systems 7,17,22. Literature reviews suggest promising results regarding the use of passive ultrasonic irrigation (PUI), as ultrasonic agitation with irrigating solutions allows for better cleaning of the root canal by removing organic tissue, bacteria, and dentinal debris 27,42. The agitation of the irrigating liquid occurs with a small file freely positioned in the root canal and close to its apical section that does not promote cuts in the dentinal walls 43.
In the field of endodont ics, dent ists are constantly searching for a more effective and faster root canal cleaning method, and the lack of protocol standardization constitutes a real problem. To date, there is no consensus regarding the time required for PUI use 6,21,31,40, and these treatment periods may vary from 10 s to 3 min 19,20,21,29. Furthermore, the influence of time on its efficacy is not clear 42. Therefore, the aim of this study was to verify the effectiveness of cleaning the canals with NaOCl and EDTA irrigation, agitated with or without an ultrasound using different treatment periods and images obtained with a scanning electron microscope (SEM).
Material and methods
The protocol followed in this study was approved by the Ethics Committee in Research of the University of Pernambuco (UPE, Camaragibe, State of Pernambuco, Brazil), under protocol #0173.0.097.000-11, ethical ly conducted in accordance with the Declaration of Helsinki (World Medical Association) by a single operator.
Selection and preparation of samples
As assessed through radiographic examination, central and lateral human mandibular incisors with intact pulp chambers and single straight canals were included in this study. Those with internal radicular resorption, external radicular resorption, or both; incompletely formed roots; pulpal calcification; or canals with curvatures exceeding 20 degrees were excluded. Forty incisors were selected and stored in a 0.1% thymol solution until use.
Crowns were removed with a diamond disc (Axis SybronEndo, Coppel, TX, USA) for standardization of the roots to 15 mm. Measurements were confirmed by a digital caliper (Digimess, São Paulo, Brazil).
A #10 K-type file (Dentsply Maillefer, Ballaigues, Switzerland) was inserted into the root canal until it was visible at the apical foramen, with the aid of a dental operating microscope (DF Vasconcelos S/A, São Paulo, Brazil) at a magnification of ×5. The working length (WL) was set at 1 mm below the apical foramen.
Instrumentation of the canals
The canals were prepared up to the F4 file of the ProTaper rotary file system (Dentsply Maillefer, Ballaigues, Switzerland), coupled to an X-Smart electric motor (Dentsply Maillefer), both of which were used according to the manufacturers' guidelines. The patency of the foramen was maintained with the #15 K-type file, inserted at each instrument change. Irrigation was performed with a syringe, a 30-gauge needle (NaviTip, Ultradent, South Jordan, UT, USA), and 3 ml of 2.5% NaOCl at each instrument change; after the end of the preparation, the canals were dried with an F4 absorbent paper point (Dentsply Maillefer).
Protocol for the final PUI irrigations
The samples were randomized into five groups with eight specimens each. The root canals were irrigated using a disposable syringe and a NaviTip 30-gauge needle (Ultradent Products®, Inc, USA), positioned 2 mm below the WL. An ultrasonic unit (ENAC, Osada, Tokyo, Japan) was used at medium power (30% to 35%) with a 21-mm IRRISAFE #25/.00 insert (Satelec, Acteon group, Merignac, France) that was positioned 2 mm below the WL. At the end of the final irrigation protocol, the canals were dried with an F4 absorbent paper point.
Control groupup
In the first step, the irrigations were performed with 3 ml of 2.5% NaOCl (Fórmula e Ação, São Paulo, SP, Brazil); with 3 ml of 17% EDTA (Fórmula e Ação) manually agitated with #15 K-type file for 3 min; and with 3 ml of physiological saline solution. In the next step, the irrigation was finalized with 3 ml of 2.5% NaOCl.
SH10 groupup with 2.5% NaOCl for 10 s
The first step was the same as in the control group; in the second step, the irrigation was concluded with 3 ml of 2.5% NaOCl using ultrasonic agitation for 10 s.
SH30 groupup with 2.5% NaOCl for 30 s
The procedures for this group were the same as in the SH10 group, except for the ultrasonic agitation, which was performed for 30 s.
SHE30 groupup with 2.5% NaOCl and 17% EDTA for 30 s
The following irrigations were performed: ultrasonic agitation with 3 ml of 2.5% NaOCl for 10 s; 3 min with 3 ml of 17% EDTA with ultrasonic agitation for 10 s; and with 3 ml of physiological saline. Subsequently, irrigation was finalized using ultrasonic agitation with 3 ml of 2.5% NaOCl for 10 s.
SHE90 groupup with 2.5% NaOCl and 17% EDTA for 90s
The procedures for this group were the same as for the SHE30 group, except for 30s of ultrasonic agitation with the solutions.
Image acquisition and analysis
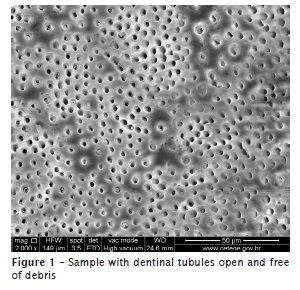
The teeth were grooved with a diamond disk and longitudinally cleaved with a rongeur that generated two hemisections, of which one was randomly chosen for use in this study. The hemisections that had failures after cutting were rejected, and new samples were selected. Samples were dried, mounted on stubs, and gold sputtered, and the images were acquired with an SEM Quanta 200F (FEI Company, Hillsboro, OR, USA), operated at 30 kV. Magnified images (×2000) were obtained and measured from the root apex, which were relative to the apical (2 mm), medial (6 mm), and cervical (12 mm) portions of the root canal. The resulting 120 images were analyzed by three evaluators, who were blinded to this study's treatments, and who had previously been calibrated in accordance with the criteria described by Rome et al. 36, which we adopted in this study. The analyses were performed by assigning scores, and the rating system was as follows: score 1 (S1), dentinal tubules were open and free of debris (figure 1); score 2 (S2), contour of the dentinal tubules was visible or partially obliterated with debris; and score 3 (S3), most contours of the dentinal tubules were imperceptible (figure 2).

Statistical analysis
The Cohen's kappa coefficient was used to calculate the concordances of inter- and intra-evaluators. For data analysis, the Kruskal-Wallis test was performed with a level of significance of 5%. SPSS v 17 (Statistical Package for the Social Sciences), and MedCalc v 12.5.0.0 were used for data input and calculations.
Results
The inter-evaluator concordance was 86% (confidence interval 0.8320–0.9814), while the intra-evaluator concordance was 97% (confidence interval 0.9632–1.0) (p = 0.9825). Therefore, according to the criteria of Landis and Koch (1977), the replicability of this study can be considered excellent (0.81–1.0).
In the cervical and medial thirds, there was no statistically significant difference regarding the cleaning according to the protocols used (p > 0.05).
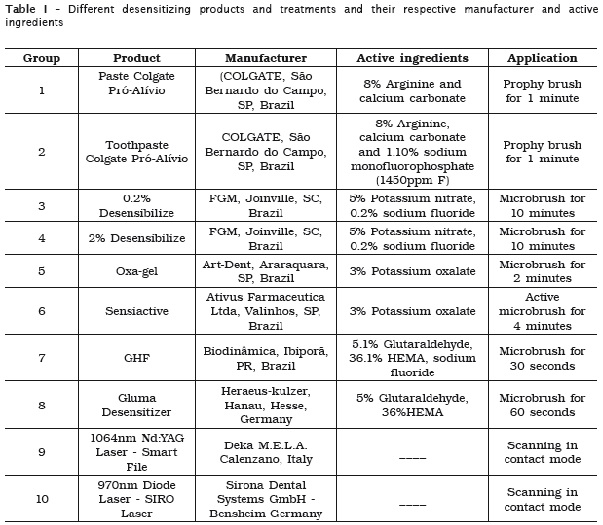
For the cleaning of the apical third, the SHE90 group showed a significant difference (p < 0.05), as compared to the control and SH10 groups. Although no significant difference, SH30 and SHE30 groups achieved superior cleaning compared to control and SH10 groups and lower than SHE90 (table 1).
Discussion
Similar to previous studies 11,14,23,25,26, the cleaning quality of the dentinal tubules after different irrigation protocols was analyzed through images obtained with SEM. In this study, the lower incisors were selected because they have an oval shape, an anatomy that clinically hampers mechanical instrumentation. Wu and collaborators 44 demonstrated that 40–60% of the inner layer of dentin remains untouched after instrumentation of the lower incisors, which have oval-shaped canals. The limitation of mechanical cleaning leads to a need for supplementation with irrigating solutions for promoting the chemical-mechanical removal of debris, as well as an antimicrobial action in areas untouched by the instruments 34. For our study, the instrumentation was performed up to the F4 file, which has a #40 diameter tip and conicity of 0.06. Brunson et al. 9 concluded that an apical preparation with a #40 diameter allows for a higher volume of irrigating fluid in the apical third, as compared to that of preparations with a lower diameter. In addition, de Gregorio et al. 13 observed that apical preparations finalized with a 40.06-diameter increase both the volume and exchange of irrigators in this same portion of the root canal.
Ultrasonic agitation with NaOCl has been proposed because it improves the cleaning quality of the root canal 5,19,28,35,38. It was used Irrisafe #25/.00 insert that has smaller diameter compared to the apical preparation to avoid touch in dentin walls during PUI. Performing ultrasonic agitation with Irrisafe tips allowed most effective debris removal and opening up dentinal tubules, especially in the apical third, comparing to conventional irrigation 28. Although this agitation does not clinically increase its antibacterial action 6, a greater penetration of NaOCl occurs in the main and lateral canals, leading to a greater reach of this solution 12. According to van der Sluis et al. 42 and Mozo et al. 27, there is a lack of agreement regarding the time required for the appropriate use of PUI. In a search for rapid and effective clinical procedures, it was decided to evaluate the proposed agitation periods in the final irrigation, including 10 s 19-21 and 30 s 30,40.
In the cervical portion, the PUI cleaning effectiveness did not have influence on the result as in Turkun and Cengiz 41 study. However, by analyzing the medium portion, these authors found superior result using PUI, disagreeing with the present study, in which PUI had similar results in all groups studied. However, in apical third, when PUI was performed with NaOCl during 10 s, the result was similar to control group which did not used ultrasonic agitation. It is possible to infer that only 10 s should not be enough to obtain a cleaning effectiveness on this root canal portion. By increasing the time to 30 s, there was a slight improvement in cleaning, but no statistical difference was observed, as it was found in Sabins et al. research 38. The same occurred when the group with NaOCl and EDTA agitation for 30 s was compared with control group. However, the agitation of the substance for a total period of 90 s increased significantly the cleaning when compared to the results found in control and SH10 groups.
The use of EDTA in the final irrigation provides more effective cleaning results in comparison with using other auxiliary chemical substances 2,23,25. In the final irrigation protocol, EDTA and NaOCl activated with PUI promoted a reduction of debris in the root canal 33. This result is consistent with the more effective cleaning of the apical third that was obtained in this study with a 30-s EDTA agitation. Al-Ali et al. 3 also observed that ultrasonic agitation with NaOCl and EDTA improved removal of the smear layer and debris from the dentinal tubules in the apical third, as compared to that of conventional irrigation usage associated only with manual agitation. Furthermore, we alternated physiological saline solution between EDTA and NaOCl applications in our study, because EDTA may interfere in the antimicrobial ability of NaOCl 37; however, the application of physiological saline is not necessary between NaOCl and EDTA usage, because NaOCl does not alter the chelating ability of EDTA 15.
Although the cleaning effectiveness may be considered more critical in the apical portion 4,8, the depth of penetration by the needle may improve the fluid distribution and the mechanical effect of an irrigating solution, with consequent debris removal 1,18. Nevertheless, Munoz and Camacho-Cuadra 30 attested that ultrasonic agitation with an irrigating liquid favored distribution in the apical third as compared to conventional irrigation, even with the needle positioned at 2 mm from the WL.
Conclusion
It can be concluded that the use of EDTA followed by NaOCl in the final irrigation protocol improves the cleaning of debris in the apical third of the root canal, whether these solutions are ultrasonically agitated for 30 s.
References
1. Abou-Rass M, Piccinino MV. The effectiveness of four clinical irrigation methods on the removal of root canal debris. Oral Surg Oral Med Oral Pathol. 1982;54:323-8. [ Links ]
2. Ahmetoglu F, Keles A, Yalcin M, Simsek N. Effectiveness of different irrigation systems on smear layer removal: a scanning electron microscopic study. Eur J Dent. 2014;8:53-7.
3. Al-Ali M, Sathorn C, Parashos P. Root canal debridement efficacy of differents final irrigation protocols. Int Endod J. 2012;45:898-906.
4. Albrecht LJ, Baumgarten JC, Marshall JG. Evaluation of apical debris removal using various sizes and tapers of ProFile GT files. J Endod. 2004;30:425-8.
5. Agrawal VS, Kapoor S. An in vitro scanning electron microscopy study comparing the efficacy of passive ultrasonic and syringe irrigation methods using sodium hypochlorite in removal of debris from the root canal system. J Ir Dent Assoc. 2012;58:156-61.
6. Beus C, Safavi K, Stratton J, Kaufman B. Comparison of the effect of two endodontic irrigation protocols on the elimination of bacteria from root canal system: a prospective, randomized clinical trial. J Endod. 2012;38:1479-83.
7. Bolles JA, He J, Svoboda KK, Schneiderman E, Glickman GN. Comparison of Vibringe, EndoActivator, and needle irrigation on sealer penetration in extracted human teeth. J Endod. 2013;39:708-11.
8. Bronnec F, Bouillaguet S, Machtou P. Ex vivo assessment of irrigant penetration and renew during the final irrigation regimen. Int Endod J. 2010;43:663-72.
9. Brunson M, Heilborn C, Johnson DJ, Cohenca N. Effect of apical preparation size and preparation taper on irrigant volume delivery by using negative pressure irrigation system. J Endod. 2010;36:721-4.
10. Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35-40.
11. Carvalho AS, Camargo CHR, Valera MC, Camargo SEA, Mancini MNG. Smear layer removal by auxiliary chemical substances in biomechanical preparation: a scanning electron microscopy study. J Endod. 2008;34:1396-400.
12. Castelo-baz P, Martín-Biedma B, Cantatore G, Ruíz-Piñón M, Bahillo J, Rivas-Mumdiña B et al. In vitro comparison of passive and continuous ultrasonic irrigation in simulated lateral canals of extracted teeth. J Endod. 2012;38:688-91.
13. de Gregorio C, Arias A, Navarrete N, Del Rio V, Oltra E, Cohenca N. Effect of apical size and taper on volume of irrigant delivery at working length with apical negative pressure at different root curvatures. J Endod. 2013;39:119-24.
14. Do Prado M, Simão RA, Gomes BPFA. Evaluation of different irrigation protocols concerning the formation of chemical smear layer. Microsc Res Tech. 2013;76:196-200.
15. Grawehr M, Sener B, Waltimo T, Zehnder M. Interactions of ethylenodiamine tetraacetic acid with sodium hypochlorite in aqueous solutions. Int Endod J. 2003;36:411-7.
16. Haapasalo M. Can I use chlorexidine as the only irrigating solution in my endodontic treatment? J Can Dent Assoc. 2011;77:b16.
17. Halford A, Ohl CD, Azarpazhooh A, Basrani B, Friedman S, Kishen A. Synergistic effect of microbubble emulsion and sonic or ultrasonic agitation on endodontic biofilm in vitro. J Endod. 2012;38:1530-34.
18. Hsieh YD, Gau CH, Kung Wu SF, Shen EC, Hsu PW, Fu E. Dynamic recording of irrigating fluid distribution in root canals using thermal image analysis. Int Endod J. 2007.
19. Jiang LM, Verhaagen B, Versluis M, van der Sluis LWM. Influence of the oscillation direction of an ultrasonic file on the cleaning efficacy of passive ultrasonic irrigation. J Endod. 2010;36:1372-6.
20. Jiang LM, Verhaagen B, Versluis M, Zangrillo C, Cuckovic D, van der Sluis LWM. An evaluation of the effect of pulsed ultrasound on the cleaning efficacy of passive ultrasonic irrigation. J Endod. 2010;36:1887-91.
21. Jiang LM, Verhaagen B, Versluis M, Langedijk J, Wesselink P, van der Sluis LW. The influence of the ultrasonic intensity on the cleaning efficacy of passive ultrasonic irrigation. J Endod. 2011;37:688-92.
22. Khan S, Niu LN, Eid AA, Looney SW, Didato A, Roberts S et al. Periapical pressures developed by nonbinding irrigation needles at various irrigation delivery rates. J Endod. 2013;39:529-33.
23. Kuah HG, Lui JN, Tseng PS, Chen NN. The effect of EDTA with and without ultrasonics on removal of the smear layer. J Endod. 2009;35:393-6.
24. McGurkin-Smith R, Trope M, Caplan D, Sigurdsson A. Reduction of intracanal bacteria using GT rotary instrumentation, 5,25% NaOCl, EDTA, and Ca(OH)2. J Endod. 2005;31:359-63.
25. Mello I, Kammerer BA, Yoshimoto D, Macedo MCS, Antoniazzi JH. Influence of final rinse technique on ability of Ethylenediaminetetraacetic acid of removing smear layer. J Endod. 2010;36:512-4.
26. Mello I, Robazza CRC, Antoniazzi JH, Coil J. Influence of different volumes of EDTA for final rinse on smear layer removal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:e40-3.
27. Mozo S, Llena C, Forner L. Review of ultrasonic irrigation in endodontics: increasing action of irrigating solutions. Med Oral Patol Oral Cir Bucal. 2012;17:e512-6.
28. Mozo S, Llena C, Chieffi N, Forner L, Ferrari M. Effectiveness of passive ultrasonic irrigation in improving elimination of smear layer and opening dentinal tubules. J Clin Exp Dent. 2014;6:e47-52.
29. Munley PJ, Goodell GG. Comparison of passive ultrasonic debridement between fluted and non-fluted instruments in root canals. J Endod. 2007;33:578-80.
30. Munoz HR, Camacho-Cuadra K. In vivo efficacy of three different endodontic irrigation systems for irigant delivery to work length of mesial canals of mandibular molars. J Endod. 2012;38:445-8.
31. Paiva SSM, Siqueira Jr. JF, Rôças IN, Carmo FL, Ferreira DC, Curvelo JA et al. Supplementing the antimicrobial effects of chemomechanical debridement with either passive ultrasonic irrigation or a final rinse with chlorhexidine: a clinical study. J Endod. 2012;38:1002-6.
32. Paqué F, Ganahi D, Peters OA. Effects of root canal preparation on apical geometry assessed by micro-computed tomography. J Endod. 2009;35:1056-9.
33. Paqué F, Boessler C, Zehnder M. Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. Int Endod J. 2011;44:148-53.
34. Rôças IN, Siqueira Jr. JF. Comparison of the in vivo antimicrobial effectiveness of sodium hypochlorite and chlorexidine used as root canal irrigants: a molecular microbiology study. J Endod. 2011;37:143-50.
35. Rödig T, Bozkurt M, Konietschke F, Hülsmann M. Comparison of the vibringe system with syringe and passive ultrasonic irrigation in removing debris from simulated root canal irregularities. J Endod. 2010;36:1410-3.
36. Rome WJ, Doran JE, Walker WA. The effectiveness of Gly-Oxide and sodium hypochlorite in preventing smear layer formation. J Endod. 1985;11:281-8.
37. Rossi-Fedele G, Doğramaci EJ, Guastalli AR, Steier L, Figueiredo JAP. Antagonistic interactions between sodium hypochlorite, chlorhexidine, EDTA, and citric acid. J Endod. 2012;38:426-31.
38. Sabins RA, Johnson JD, Hellstein JW. A comparison of the cleaning efficacy of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canals. J Endod. 2003;29:674-8.
39. Siqueira Jr. JF, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008;34:1291-301.
40. Spoorthy E, Velmurugan N, Ballal S, Nandini S. Comparison of irrigant penetration up to working length and into simulated lateral canals using various irrigating techniques. Int Endod J. 2013;46:815-22.
41. Türkün M, Cengiz T. The effects of sodium hypochlorite and calcium hydroxide on tissue dissolution and root canal cleanliness. Int Endod J. 1997;30:335-42.
42. van der Sluis LWM, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40:415-26.
43. Weller RN, Brady JM, Bernier WE. Efficacy of ultrasonic cleaning. J Endod. 1980;6:740-3.
44. Wu MK, van der Sluis LWM, Wesselink PR. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int Endod J. 2003;36:218-24.
45. Zehnder M. Root canal irrigants. J Endod. 2006;32:389-98.
 Corresponding author:
Corresponding author:
Felipe Xavier
Avenida Gal Newton Cavalcanti, n. 1.650 – Tabatinga
CEP 54753-901 – Camaragibe – PE – Brasil
E-mail: felipefatah@hotmail.com
Received for publication: May 5, 2014
Accepted for publication: September 1, 2014













