Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.4 Joinville Out./Dez. 2014
ORIGINAL RESEARCH ARTICLE
Evaluation of correlation among sleep bruxism and depression levels, chronic pain and nonspecific physical symptoms according to axis II of the Research Diagnostic Criteria/Temporomandibular disorders
Isabela Maddalena Dias I; Ingrid Duque Maia II; Lívia Marins Ramalho de Mello II; Isabel Cristina Gonçalves Leite III; Fabíola Pessôa Pereira LeiteIV
I School of Medicine, Federal University of Juiz de Fora – Juiz de Fora –MG – Brazil
II School of Dentistry, Federal University of Juiz de Fora – Juiz de Fora – MG – Brazil
III Department of Collective Health, School of Medicine, Federal University of Juiz de Fora – Juiz de Fora – MG – Brazil
IV Department of Restorative Dentistry, School of Dentistry, Federal University of Juiz de Fora – Juiz de Fora – MG – Brazil
ABSTRACT
Introduction: Sleep Bruxism (SB) is considered as a parasomnia and defined as a stereotyped movement disorder characterized by grinding (eccentric), hitting or shaking (central) of teeth while sleeping unconsciously, and classified according to their etiology in primary (idiopathic) or secondary (associated with medical or psychiatric conditions) of multifactorial etiology. Certain risk factors such as alcohol/tobacco, caffeine, use of certain medications, conditions associated with sleep, psychological factors, among others, may trigger or enhance certain oral parafunctions. Objective: The present study aimed to observe statistically the existence of a possible correlation between SB and psychological aspects studied by the Research Diagnostic Criteria/Temporomandibular disorders (RDC/TMD): chronic pain, depression, nonspecific physical symptoms (NSPS) including pain, NSPS excluding pain. Material and methods: 50 patients with SB and 49 patients without SB were assessed, aged between 18 and 70 years at the TMD/Orofacial Pain Clinics of the School of Dentistry, Federal University of Juiz de Fora. These individuals were investigated by RDC Axis II on the severity levels of chronic pain, depression, nonspecific physical symptoms (NSPS) including pain, NSPS excluding pain, to verify the correlation of these variables with the SB. Results: There was a statistically significant correlation between BS and chronic pain severity (p = 0.001), and SB and NSPS including pain (p = 0.026), and SB and NSPS excluding pain (p = 0.018). There was no significant correlation between SB and depression. Regarding the severity of chronic pain, there was a higher prevalence of grade 2 (79.60%) in patients with BS and grade 1 (52%) in the patients without SB. According to the other assessed levels, a greater severity of psychological aspects evaluated by RDC/TMD were seen in patients with BS and more normal levels in patients without SB. Conclusion: Levels of chronic pain severity, nonspecific physical symptoms with or without pain (somatization) appeared as aspects involved in sleep bruxism. These findings emphasize the importance of an accurate assessment of the parafunction etiology for each case, which often requires a multidisciplinary approach.
Keywords: sleep bruxism; chronic pain; somatization; depression.
Introduction
The stomatognathic system composed by interrelated structures as teeth, muscles, periodontium, ligaments and temporomandibular joints (TMJs) accounts for performing essential functions as fonation, swallowing, and mastication. Notwithstanding, some activities classified as parafunctional, that is, of abnormal pattern, but constantly executed by many individuals, overload the masticatory system. During the day, one of these activities can be characterized by tightening the teeth together with other habits injurious to health (biting of tongue, cheeks, and foreign objects; thumb sucking), which can even be related to the professional activity of the patient. According to the literature, parafunctional activity frequently occurs during sleeping, e.g., sleep bruxism 14,21,26,28.
Bruxism is one of the most prevalent, complex, and destructive orofacial disorders. The name is originated from the Greek expression brychein odontas ("teeth grinding"), and it is the term used for either static or dynamic contact of teeth occlusion during moments other than those during the normal mastication and/or swallowing functions and thus considered as a parafunctional habit. Sleep bruxism can be either centric (act of tightening) and/or eccentric (act of gridding) and may occur at either the day or the night. Sleep bruxism is classified as parasomnia and defined as a stereotyped movement disturb characterized by the grinding, hitting, or tightening of the teeth, unconsciously and involuntarily, generally associated with sleep physiology. It can be also classified according to its etiology as primary and secondary. Primary or idiopathic bruxism is of unknown cause; secondary bruxism may occur associated with many medical or psychiatric conditions 4,14,15,18,24,26,28,34.
Because of its involuntary and unconscious condition, sleep bruxism occurrence may be underestimated, which makes difficult to obtain reliable estimates of its prevalence. It is known that sleep bruxism decreases with age. According to some studies, sleep bruxism is found in 3.5% to 40.6% of the children, in 12.8% of adults, and in 3% of individuals above 60 years-old 19,20,28,29,32.
Sleep bruxism etiology has been frequently studied and seems to be of multifactorial etiology, including risk factors of alcohol/smoke consumption, excessive use of caffeine, use of some medicaments (fluoxetine, paroxetine, and sertraline), upper airway diseases, conditions associated with sleep, psychological factors, pain state (especially chronic), among others 2,5,15,16,21,31,25-37.
A close relationship between psychic stress and bruxism has been established by most of the studies on bruxism, and many neurophysiological studies have concerned about bruxism occurrence and etiology. The psychosocial variables, such as emotional stress, depression, and anxiety play a preponderant role in sleep bruxism pathogenesis, both at the perpetuation, treatment, frequency, duration and severity of this parafunction 10,16. According to Carvalho et al. 10, the emotional alterations, such as anxiety and stress, may be related to the increasing of muscle tone, because of the central nervous system (CNS) stimulation to daily stressful events.
Carlsson et al. 8 affirmed that a theory strongly accepted by most of the researchers is that sleep bruxism is a disorder related to the emotional conditions of the patient. The bruxism occurrence may vary according to more stressful days and the anxiety related to important events in the patient's life.
Patients exhibiting sleep bruxism may frequently report some symptoms mainly especially when waking, such as headache in temporal area, stiff or fatigued mandibular muscle, locked mouth opened or difficult in opening the mouth, difficult in moving the mandible, and even tooth hypersensitivity. During the cleaning evaluation of sleep bruxism, sleep noises are frequently reported by either the patient or the relatives. It is noted a considerable esthetic impairment because of tooth wearing, restoration/tooth fracture, abfraction, recessions, and tooth marks on tongue 4,19,34,38. Bruxism has been considered as one of the risk factors for the occurrence or worsening of temporomandibular disorders 14,30.
Considering that some psychological factors many act by triggering or perpetuating oral parafunctions and these latter are very harmful to the masticatory system, it is noteworthy to investigate this possible correlation. This study aimed to verify the correlation through evaluating the depression levels, chronic pain and somatization severity (nonspecific physical symptoms) in patients with or without sleep bruxism.
Material and methods
To conduct this cross-sectional study, 99 patients (83 females and 16 males), aged from 18 and 70 years (mean age of 43.4 years) were evaluated in the Temporomandibular Disorder/Orofacial Pain Clinics of the School of Dentistry, Federal University of Juiz de Fora. This present study was submitted and approved by the Ethical Committee in Research of the institution under protocol no. #310.561.
All study participants signed a Free and Clarified Consent form. Firstly, the patients were evaluated regarding to the clinical diagnosis of sleep bruxism through the criteria of Lavigne et al. 20 and Lavigne and Manzini 21, who combined at least two of the following clinical findings:
1) History of grinding or shear noises of teeth confirmed by other person;
2) Detection of tooth weariness not compatible with that from age and/or normal function;
3) Headache on temporal area;
4) Stiff or fatigued mandibular muscle at night or wakening;
5) Locking or difficult in opening the mouth at the morning;
6) Tooth hypersensitivity;
7) Hypertrophy of masseter muscles. The exclusion criteria adopted was the presence of neurological diseases because they may trigger secondary bruxism associated with some medical cause, which was not the aim of this present study.
The patients were divided into two groups: patients diagnosed with sleep bruxism (group I – 50 individuals [44 females and 6 males]) and patients without diagnosis of sleep bruxism (group II – 49 individuals [39 females and 10 males]). Sample size calculation was based on the estimated prevalence of bruxism in the adult population according to the literature (12.8%) and in the number of patients treated in the institutional clinics per semester. Firstly, both groups had 50 patients. However, because of errors in the filling of one study charts, the data of one patient without sleep bruxism were excluded.
All patients were submitted to the evaluation of chronic pain severity, levels of depressive symptoms, and nonspecific physical symptoms (including or excluding pain) through Research Diagnostic Criteria (RDC) axis II 12,23. The scores regarding to the psychological evaluation were classified in normal, moderate, and severe.
RDC development aimed at establishing reliable and valid criteria to diagnosis and defined subtypes of muscle and articular diseases that may occur in the masticatory system, classified as temporomandibular disorder. Notwithstanding, RDC contains a 10-item form for physical examination to diagnose the disorder type of the patient and the axis II comprising a self-report questionnaire with 31 questions that enable to classify each case according to the psychological conditions of the patient (intensity and incapacity of the chronic pain severity, depression degree, and nonspecific physical symptoms) 12,23,33.
The obtained data was evaluated through Spearman's coefficient, with level of significance of 5% because this test allowed evaluating the correlation among the ordinal variables and those used in the study.
Results
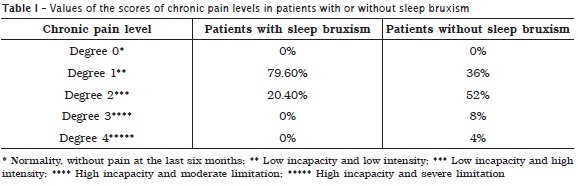
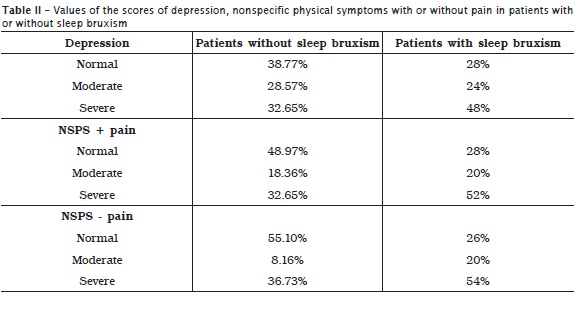
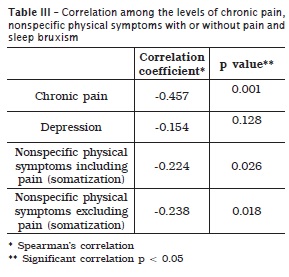
Tables 1 and 2, respectively display the scores of incapacity and severity of chronic pain in patients with and without sleep bruxism and the prevalences corresponding to the depression levels and nonspecific physical symptoms including or excluding pain. A higher prevalence of degree 1 pain was noted in patients without sleep bruxism, while a higher prevalence of degree 2 pain was seen in patients with sleep bruxism. Concerning to depression levels and nonspecific physical symptoms including or excluding pain, a higher prevalence of normal levels occurred in the group of patients without sleep bruxism. In the group of patients with sleep bruxism, it could be noted a considerable severe levels of depression and nonspecific physical symptoms including or excluding pain. According to table 3, it could be verify a statistically significant correlation among sleep bruxism, incapacity and severity of chronic pain, and severity of nonspecific physical symptoms.
Discussion
Bruxism is considered as the most damaging physiopathological disturb among all parafunctional activities of the stomatognathic system, at any age range and in both sexes, with multifactorial etiology. The combination of external and psychic factors seems to account for this type of parafunction. It is characterized by a repetitive, involuntary activity, including either the tightening (centric) or grinding (eccentric) of teeth and/or mandibular boosting. It can happen during the day (daytime bruxism) or during the sleep (sleep bruxism) 14,15,17,26,28.
Sleep bruxism is an unconscious activity of grinding (eccentric), hitting, or tightening (centric) of teeth during sleep, classified as a parasomnia of primary character when it is not related to any apparent cause; and secondary when it is associated to medical and psychiatric factors 14,15,26.
According to Alencar Jr. 4, the normal adult sleep has a clearly defined architecture, staged based on the frequency and amplitude of the electroencephalographic activity. The sleep is divided into REM (rapid eye movements) and NREM sleep (not REM). Not REM sleep (synchronized sleep) is classified at stages 1, 2, 3, and 4, and the first two stages comprise superficial sleep. In adults, the episodes of sleep bruxism have been frequently associated with micro-awakenings at the superficial sleep stages (1 and 2), with greater prevalence in superficial sleep than in deep sleep. Bruxism etiology is still controversial and many studies pointed out a multifactorial etiology. Among the factors associated with sleep bruxism, the literature emphasized those related to stress, anxiety, behavioral and cognitive factors. The emotional alterations, such as anxiety and stress may be associated with sleep bruxism because when stressful events are present they are significant risk factors due to CNS stimulation, increasing the muscle activity both during daytime and sleep 3,6,13,17,26,27.
Santos et al. 36 highlighted that CNS alterations have a significant relationship with sleep bruxism etiology. Carlsson et al. 8 suggested that sleep bruxism has central and non-peripheral regulation, emphasizing that during bruxism episodes, the brain is firstly activated and then an autonomic cardiac acceleration is noted, promoting a strong activation of the masticatory muscles. On the other hand, according to Manfredini and Lobbezzo 26, additionally to the stress, other symptoms such as the anxiety, depression, psychosomatic disturbs, emotional stress, and personality disorders show high and significant prevalence among individuals with bruxism.
In this present study, we aimed to verify the correlation between sleep bruxism and psychological aspects studied by RDC/TMD 12,23 (chronic pain, depression, NSPS including or excluding pain). A significant correlation was found between sleep bruxism and chronic pain severity (p = 0.001). This result may be justified by the fact that the evaluated patients were undergoing treatment with main complaints of chronic orofacial pains 4,9,14,34.
Also, a correlation between sleep bruxism and NSPS including pain was seen (p = 0.026) and sleep bruxism and NSPS excluding pain (p = 0.018). This finding may also be justified by the fact that TDM patients have high levels of somatization, according to the literature 26,30.
No significant correlation was seen between sleep bruxism and depression (p = 0.128), disagreeing with the literature 17,25. The rationale behind this result is that the RDC axis II is not specific for the assessment of depression.
Fissmer et al. 15 pointed out that Dentistry undergraduate students presenting anxiety exhibited chances four times greater of having bruxism when compared with those without anxiety. Endo et al. 13 highlighted that daytime tooth tightening is associated with psychological factors, mainly anxiety. In this present study, however, the evaluation of the relationship between sleep bruxism and anxiety was not possible because RDC axis II do not assess the levels of anxiety of the patients. Gungormus and Erciyas 17 found that the anxiety and depression mean scores of patients with bruxism was higher than those of patients without bruxism. Manfredini et al. 25 also emphasized that individuals with bruxism are more depressed and anxious than those without bruxism. Notwithstanding, in 2011, Manfredini et al. 27 reported that the role of the personality features of the patients may be more important than depression, for example, regarding to the activity of masticatory muscles during sleep. In this present study, no statistically significant correlation was verified between sleep bruxism and depression (p = 0.128).
The study of Manfredini and Lobbezzo 26 demonstrated that additionally to stress, other symptoms as anxiety, depression, psychosomatic disturbs, emotional stress, psychiatric disorders (schizophrenia) and personality traits had higher and significant prevalence in individuals without bruxism. According to Fernandes et al. 14, the sleep bruxism and temporomandibular disorders increase the chance of an individual exhibits nonspecific physical symptoms, characterizing the sleep bruxism as an important risk factor. In this present study, a significant correlation between sleep bruxism and psychological/psychosomatic factors as nonspecific physical symptoms excluding pain (p = 0.018) and nonspecific physical symptoms including pain (p = 0.026).
Still in relation to the nonspecific physical symptoms, the systematic review of Coelho and Ávila 11 on somatization verified that the individual has to experience and tends to communicate the anxieties somatically, that is, through physical symptoms that do not have a pathological evidence (clinically undefined), but the individual attributes to organic diseases and seeks medical aid. Generally, somatization is manifested in response to psychosocial stresses such as life events with a common symptom. The theory that sleep bruxism has central and non-peripheral regulation, emphasized by Lobbezzo and Naeije 22, agrees with those reports. In the study of Camparis et al. 7, all patients with sleep bruxism also showed high levels of "severe" somatization (p = 0.001). The study of Bayar et al. 6 also exhibited that there is a difference in the levels of somatization of patients with and without sleep bruxism. Corroborating these findings, in this present study, we verified that 52% of the patients had severe level of non-specific physical symptoms excluding pain and 54% of the patients had severe level of non-specific physical symptoms including pain.
According to Seraidarian et al. 37, pain state, mainly chronic, has been described by the literature as a factor associated with bruxism. The results of this present study corroborate this finding because we verified a statistically significant correlation between sleep bruxism and chronic pain (p < 0.001). It is noteworthy to mention that 52% of the patients with sleep bruxism exhibited score 2 of chronic pain (low incapacity and high intensity) and only 20.4% of the patients without sleep bruxism had this score in chronic pain assessment.
Abekura et al. 1 found that the mean salivary CgA levels were significantly increased after a stressful task, which did not occur in patients without bruxism. This finding was also seen in the study of Carvalho et al. 10, who verified a stress in 3.7% of non-bruxers and in 33.3% of bruxers. The authors highlighted that the psychosocial variables, such as emotional stress, depression and anxiety play a preponderant role in sleep bruxism pathogenesis, during the onset, perpetuation, treatment, frequency, duration and severity.
According to the results of this present study, it could be verified that some emotional aspects were correlated with sleep bruxism. This finding emphasizes the importance of not only the symptomatic treatment (mainly through stabilizing plate and drugs), but also the necessity of reducing or eliminating the patient exposure to some psychological factors.
Conclusion
• Degree 2 chronic pain (low incapacity and high intensity) was frequently found in 52% of patients with sleep bruxism and only in 20.4% of patients without sleep bruxism;
• None patients without sleep bruxism exhibited degree 4 chronic pain (High incapacity and severe limitation) while 4% of patients with sleep bruxism exhibited degree 4 chronic pain;
• Depression was found at severe level in 48% of patients with sleep bruxism and in 32.65% of patients without sleep bruxism;
• NSPS including or excluding pain, at severe degree, was found in 52% and 54%, respectively, of patients with sleep bruxism. In patients without sleep bruxism, these rates were smaller: 32.65% and 36.73%, respectively;
• Levels of chronic pain severity, non-specific physical symptoms including or excluding pain (somatization) showed correlation with sleep bruxism.
References
1. Abekura H, Tsuboi M, Okura T, Kagawa K, Sadamori S, Akagawa Y. Association between sleep bruxism and stress sensitivity in an experimental psychological stress task. Biomedical Research. 2011;32(6):395-9. [ Links ]
2. Ahlberg K, Ahlberg J, Könönen M, Partinen M, Lindholm H, Savolainen A. Reported bruxism and stress experience in media personnel with or without irregular shift work. Acta Odontolol Scand. 2003;61(5):315-8.
3. Ahlberg J, Lobbezoo F, Ahlberg K, Manfredini D, Hublin C, Sinisalo J et al. Self-reported bruxism mirrors anxiety and stress in adults. Med Oral Patol Oral Cir Bucal. 2013;18(1):7-11.
4. Alencar Jr. FPG. Oclusão, dores orofaciais e cefaléias. São Paulo: Santos; 2005.
5. Bader G, Lavigne G. Sleep bruxism: an overview of an oromandibular sleep movement disorder. Sleep Med Rev. 2000;4(1):27-43.
6. Bayar GR, Tutuncu R, Acikel C. Psychopathological profile of patients with different forms of bruxism. Clin Oral Invest. 2012;16(1):305-11.
7. Camparis CM, Siqueira JT. Sleep bruxism: clinical aspects and characteristics in patients with and without chronic orofacial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(2):188-93.
8. Carlsson GE, Magnusson T, Egermark I. Prediction of demand for treatment of temporomandibular disorders base on a 20-year follow-up study. J Oral Rehabil Oxford. 2004;31(6):511-7.
9. Carlsson GE, Magnusson T, Guimarães AS. Tratamento das disfunções temporomandibulares na clínica odontológica. São Paulo: Quintessence; 2006.
10. Carvalho AL, Cury AA, Garcia RC. Association between bruxism and emotional stress in military policemen. Rev Odonto Ciênc. 2008;23(2):125-9.
11. Coelho CLS, Ávila LC. Controvérsias sobre a somatização. Rev Psiq Clín. 2007;34(6):278-84.
12. Dworkin SF, Le Resche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. Journal Craniomandibular Disorders. 1992;6(4):301-55.
13. Endo H, Kanemura K, Tanabe N, Takebe J. Clenching occurring during the day is influenced by psychological factors. Journal of Prosthodontic Research. 2011;55(3):159-64.
14. Fernandes G, Franco AL, Siqueira JT, Gonçalves DA, Camparis CM. Sleep bruxism increases the risk for painful temporomandibular disorder, depression and non-specific physical symptoms Journal of Oral Rehabilitation. 2012;39(7):538-44.
15. Fissmer JFW, Garanhani RR, Sakae TM, Traebert JL, Soar Filho EJ. Relação entre ansiedade e bruxismo em acadêmicos de Odontologia. Arquivos Catarinenses de Medicina. 2008;37(1):25-9.
16. Gomez FM, Ortega JE, Horrillo I, Meana JJ. Relationship between non-functional masticatory activity and central dopamine in stressed rats. J Oral Rehabil. 2010;37(11):827-33.
17. Gungormus Z, Erciyas k. Evaluation of the relationship between anxiety and depression and bruxism. The Journal of International Medical Research. 2009;37(2):547-50.
18. Kato T, Thie NMR, Montplaisir JY, Lavigne GJ. Bruxism and orofacial movements during sleep. Dent Clin North Am. 2001;45(4):657-84.
19. Kato T, Thie NMR, Huynh N, Miyawaki S, Lavigne GJ. Topical review: sleep bruxism and the role of peripheral sensory influences. J Orofac Pain. 2003;17(3):191-213.
20. Lavigne GJ, Rompré PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75(1):546-52.
21. Lavigne GJ, Manzini C. Principles and practice of sleep medicine. Philadelphia: WB Saunders; 2000.
22. Lobbezoo F, Naeije M. Bruxism is mainly regulated centrally, not peripherally. J Oral Rehabil. 2001;28(12):1085-91.
23. Lucena LB, Kosminsky M, Costa LJ, Góes PS. Validation of the Portuguese version of the RDC/TMD Axis II questionnaire. Braz Oral Res. 2006;20(4):312-7.
24. Macedo CR, Silva AB, Machado MA, Saconato H, Prado GF. Oclusal splint for treating sleep bruxism (tooth grinding). Cochrane Database Syst Rev. 2007;17(4):18-27.
25. Manfredini D, Landi N, Romagnoli M, Bosco M. Psychic and occlusal factors in bruxers. Aust Dent J. 2004;49(2):84-9.
26. Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain. 2009;23(2):153-66.
27. Manfredin ID, Fabbri A, Peretta R, Guarda-Nardini L, Lobbezoo F. Influence of psychological symptoms on home-recorded sleep-time masticatory muscle activity in healthy subjects. J Oral Rehabil. 2011;38(12):902-11.
28. Manfredini D, Restrepo C, Diaz-Serrano K, Winocur E, Lobbezoo F. Prevalence of sleep bruxism in children: a systematic review of the literature. J Oral Rehabil. 2013;40(8):631-42.
29. Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain. 2013;27(2):99-110.
30. Mora MS, Weber D, Borkowski S, Rief W. Nocturnal masseter muscle activity is related to symptoms and somatization in temporomandibular disorders. J Psychosomatic Res. 2012;73(4):307-12.
31. Moraes MSBF, Oliveira NM. Bruxismo. Rev Faculdade de Ciências Médicas de Sorocaba. 2006;8(2):5-6.
32. Ohayon MM, Li KK, Guilleminault C. Risk factors for sleep bruxism in the general population. Chest Northbrook. 2001;119(1):53-61.
33. Ohrbach R. Assessment and further development of RDC/TMD axis II biobehavioural instruments: a research programme progress report. J Oral Rehabil. 2010;37(10):784-98.
34. Okeson JP. Tratamento das desordens temporomandibulares e oclusão. Rio de Janeiro: Elsevier; 2008.
35. Pereira RPA, Negreiros WA, Scaparo HC, Pigozzo MN, Consani RLX, Mesquita MF. Bruxismo e qualidade de vida. Rev. Odonto Ciência. 2006;21(52):185-90.
36. Santos AAR, Bergantin AG, Maekawa MY, Maekawa LE, Marcacc S. Análise crítica da participação dos fatores odontológicos e psicológicos na etiologia do bruxismo. Revista Odontológica de Araçatuba. 2007;28(2):20-4.
37. Seraidarian PI, Assunção ZLV, Jacob MF. Bruxismo: uma atualização dos conceitos, etiologia, prevalência e gerenciamento. JBA. 2001;1(4):290-5.
38. Winocur E, Hermesh H, Littner D, Shiloh R, Peleg L, Eli I. Signs of bruxism and temporomandibular disorders among psychiatric patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(1):60-3.
 Corresponding author:
Corresponding author:
Isabela Maddalena Dias
Rua Espírito Santo, n. 1.387 – Centro
CEP 36016-200 – Juiz de Fora – MG – Brasil
E-mail: isabelamdias@gmail.com
Received for publication: February 28, 2014
Accepted for publication: May 22, 2014













