Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.4 Joinville Out./Dez. 2014
ORIGINAL RESEARCH ARTICLE
In vitro reduction of Streptococcus mutans biofilm on silver nanoparticle-modified composite resin
Patrícia Bolzan Agnelli das Neves I; Clovis Wesley Oliveira de Souza I; Elisabeth Loshchagin Pizzolitto II
I Department of Morphology and Pathology, Federal University of São Carlos – São Carlos – SP – Brazil
II Department of Clinical Analysis, School of Pharmaceutical Sciences, Sao Paulo State University – Araraquara – SP – Brazil
ABSTRACT
Introduction: Currently, new methods to reduce biofilm formation on biomaterials are very studied, for example the use of silver nanoparticles, which were bactericidal. However, there are few studies investigating the benefits of these particles in dental restorative materials. Objective: This study aimed to compare in vitro the Streptococcus mutans biofilm formation on conventional light-cured composite resin with that on experimental light-cured composite resin, modified with silver nanoparticles. Material and methods: Discs were produced with either conventional resin (control group) and resin modified with different concentrations of silver nanoparticles, 0.1%, 0.3% and 0.6 % wt. (groups 1, 2 and 3, respectively). The samples were incubated in bacterial suspension (S. mutans) enriched with 20% sucrose to promote biofilm growth on the surfaces. Incubation times were 1, 4 and 7 days. After each period, adherent biofilms were disaggregated by ultrasound. Then, the numbers of viable cells recovered from the biofilms were counted through the serial dilution method. A morphological analysis of biofilm was also performed by Scanning Electron Microscopy. The data were subjected to Anova and Tukey's test (α = 0.05). Results: The number of viable cells was statistically lower in groups 2 and 3 than in group 1 and control group, after the three incubation periods, without statistical difference between groups 2 and 3. The number of viable cells was statistically lower in group 1 than in control group, after 4 and 7 days of incubation. Conclusion: Resins modified with silver presented reduction of S. mutans biofilm on their surfaces, according to the conditions of this study.
Keywords: biofilms; Streptococcus mutans; composite resins; nanoparticles.
Introduction
Bacterial biofilm can be simply defined as a set of microorganisms and their extracellular products, adhered on many surface types. Although microorganisms can live free in the environment, most bacteria are associated in a biofilm. Biofilms make easy to obtain nutrients and increase the resistance to antibiotics 10. In many situations, biofilm formation is harmful to human beings causing diseases. To discover methods of reducing the biofilm growth on some materials is challenging both for the science and industry 9.
The antimicrobial activity of some materials show when nanoparticles are added has been recently demonstrated and largely studied as the new method to allow controlling the microbial biofilm growth on surfaces 8,17,18. Chitosan, silver, ZnO and TiO2 nanoparticles have been studied in coatings or incorporated to materials, especially biomaterials and food packages, to obtain products less favorable to microbial proliferation 2,11,16,24. Silver nanoparticles have already been commercially available as an antimicrobial component in parts of household appliances, such as air conditioners, air fresheners, water fresheners, and hair dryers 17,18,26.
Bacterial biofilm formation causes many diseases inside oral cavity: gingivitis, periodontal disease, and caries, so that the search for alternative methods for biofilm control are very important 6,28,30. Not only the teeth, but also the restorative material can be colonized by bacteria and allow biofilm growth 3,7,12. Thus, innovations enabling the control of biofilm growth on such materials have been the aim of many researches, for example, the use of silver nanoparticles and other alternative compounds 4,13,15,25. A light-cured resin composite with antimicrobial activity of the surface, but without damaging to the physical properties, may help in secondary caries prevention, that is, those occurring around restorations or in tooth/ restoration interface, because resin composite is one of the material most employed in restorations, nowadays 19,21,22.
Some in vitro studies evaluated resin composite modified by silver 1,5. The results are encouraging. Bürgers et al. 5 evaluated the antimicrobial activity of a resin composite containing silver nanoparticles, at two concentrations, and verified that the amount of adhered bacterial cells was smaller. Ahn et al. 1 compared two conventional resin bonding agents with one bonding agent containing silver nanoparticles and also verified that the number of adhered bacterial cells was smaller on the experimental bonding agent.
In the light of this, this study aimed to evaluate quantitatively the Streptococcus mutans biofilm formation (the main species accounting for caries onset) adhered on the surfaces of both conventional and silver-modified resin composites, through the counting of viable cells recovered from the biofilms. Also, we aimed to evaluate the morphology of the biofilm formed on both materials through scanning electronic microscopy.
Material and methods
Sample construction
Discs of conventional (control group) and silver nanoparticle-modified resin composite at three different concentrations of 0.1%, 0.3%, and 0.6% (groups 1, 2, and 3, respectively), by weight were constructed. The resin composite was Filtek Z250®, (3M-ESPE, Maplewood, MN, EUA). The silver nanoparticle-base additive was Nanox Clean® (Nanox Tecnologia S.A., São Carlos, SP, Brazil).
To construct the modified resin composite discs, previously portions of 1 g of resin and portions of the necessary amount of additive were weighted to obtain the desired concentration. The silver nanoparticle additive was incorporated into the resin composite by mixing both materials manually for one minute.
Cylindrical plastic matrixes measuring 4 mm in diameter and 4 mm in height were used to construct the resin composite discs to standardize both the shape and dimension of all samples. First, a 2-mm increment of resin composite was placed inside the matrix and light-cured for 20 s. Then, the matrix was completely filled and the resin composite light-cured again. After that, the resin composite disc was removed from the matrix. The portion of resin composite mixed with the silver particles was stored into a black box until use to avoid the spontaneous light-curing by natural light and early setting. After the filling of the matrix by the modified resin, the next increment of modified resin was removed from the storage box to obtain a new disc.
The light-curing device used (Optilight LD III, Gnatus, Ribeirão Preto, SP, Brasil), with power of 15 VA and frequency of 50 Hz. Following, all sample surfaces were polished with the aid of silicone points (Enhance, Dentsply, Adlestone, Surrey, UK) and sandpaper (Sof-Lex, da 3M ESPE, Maplewood, MN, USA). Then the samples were individually packed according to the experimental group and sterilized by autoclave (121ºC, 1 atm) for 15 min.
In vitro biofilm formation and counting of viable cells
Streptococcus mutans strain (ATCC 25175, from Oswaldo Cruz Foundation), was cultivated in brain heart infusion broth (BHI) for 24 h, and then a standardized bacterial suspension was prepared. The culture was centrifuged at 3,000 rpm for 20 min (Excelsa II, model 206-B, FANEM, São Paulo, SP, Brazil). The supernatant was discarded and the biomass was precipitated and resuspended in phosphate buffered saline solution (PBS), enriched with 20% sucrose, previously sterilized until reaching an absorbance of 0.08, corresponding to a number of cells of 108 CFU/ml, measured under 600 nm wavelength, with the aid of spectrophotometer (FEMTO – São Paulo, SP, Brazil).
The resin composite discs were incubated in 24-well polystyrene microplate (Nunclon TM, Nunc, Thermo Scientific), at 37ºC. Each well was filled with 2 ml of S. mutans standardized suspension in PBS, to allow the biofilm formation adhered on the samples. The incubation time periods were 1, 4, and 7 days. To this study, six discs were produced for each experimental group (n = 72). The experiment was performed in triplicate and repeated twice (six repetitions).
Elapsed each incubation time, the medium inside the wells was removed by pipette and the discs were washed in PBS, three times, to remove the weakly adhered cells. Then, the discs were transferred into vials containing 10 ml of PBS and took to an ultrasound device (Digital Ultrasonic Cleaner, Kondortech, São Carlos, SP, Brazil) at cleaning power of 160 W, for 8 min to dissociate the biofilm.
The viable cells after sonication were counted through serial dilution method. From the initial solution, five sequential solutions were obtained. 100 μL of each solution was pipetted into Petri plates containing BHI agar culture medium, with 20% sucrose. The inocula were spread with the aid of Drigalski spatula and the plates were incubated at 37ºC, for 48 h, to enable the bacterial proliferation and the appearance of the colonies visible to naked eye. The plates containing from 30 to 300 colonies were chosen for the counting of the number of colonies, corresponding to the number of colony forming unity (CFU), or viable bacterial cells. CFU value was multiplied by the dilution factor to obtain the value of cells in the initial solution and then divided by 10 to obtain CFU/ml.
The mean and standard deviation of the six values of each group and incubation time (n = 6) was recorded. Data was statistically analyzed by Anova and Tukey test.
Biofilm morphologic analysis
For this analysis, three discs were constructed for each experimental group (n = 12). The discs were also incubated in PBS + sucrose containing S. mutans inside polystyrene well plate. The disc surfaces were observed after the three incubation times for biofilm formation: 1, 4, and 7 days. Elapsed each period, the culture media were removed from the wells and the discs were washed by sterilized PBS, three times, with the aid of automatic pipettes. The biofilm fixation process was as follow: immersion in 20% glutaraldehyde for 15 min; progressive immersion in a series of alcoholic solutions at increasing concentrations (15, 30, 50, 70, 90, and 100%) for 15 min each one; drying at environment temperature for 12 hours. Then, the resin composite discs were fixed into a metallic device of the microscope with metallic glue and covered by gold, inside a vacuum chamber, to obtain a conducting surface required for scanning electronic microscopy (model FEG XL30, FEI Company, Hillsboro, OR, USA).
Results
Viable cell counting
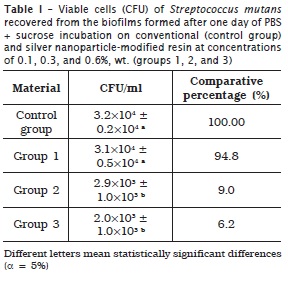
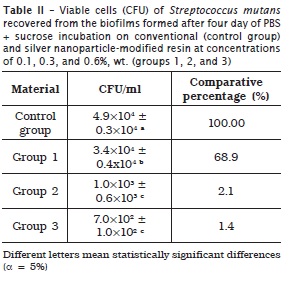
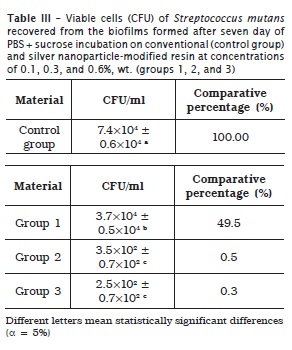
Tables 1, 2 and 3 display the means and standard deviations of the number of viable cells recovered from the biofilms for the four experimental groups, at three incubation periods.
At one day, groups 2 and 3 exhibited CFU/ml value smaller than that of group 1 and control group, without statistically significant differences (p > 0.05). At four days, group 1 showed CFU/ml value smaller than that of control group and that of groups 2 and 3 (statistically similar), with statistically significant differences (p < 0.05). At seven days, group 1 presented CFU/ml value smaller than that of control group, and that of groups 2 and 3 (statistically similar), with statistically significant differences (p < 0.05).
Biofilm morphologic analysis
Figure 1 shows some SEM images of biofilm formation on the surfaces of the samples incubated for one day. The control group exhibited the plentiful presence of bacterial cells adhered to either the surface or among each other. Similar aspects were seen in group 1 samples Group 2 and 3 samples showed a smaller number of cells than did control group.
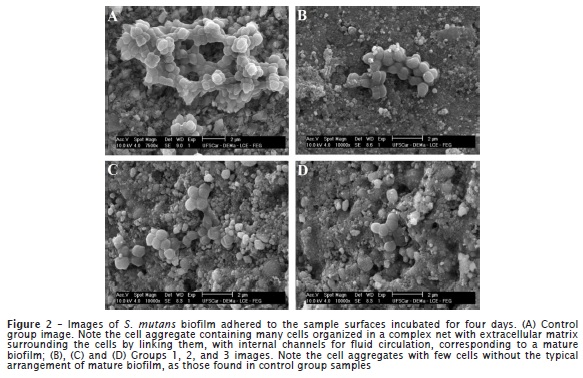
Figure 2 displays the surface images of the samples incubated for four days. Control group samples exhibited many cells of complex aggregation with extracellular matrix surrounding the cells by linking them. The cells were grouped in structures containing cell branches and internal channels for fluid circulation, which is compatible with a mature biofilm. Groups 1, 2 and 3 did not show mature biofilm with few cell groups on the surfaces. Figure 3 highlights the presence of the large extracellular matrix in some of the mature biofilm on the conventional resin composite after four days of incubation.
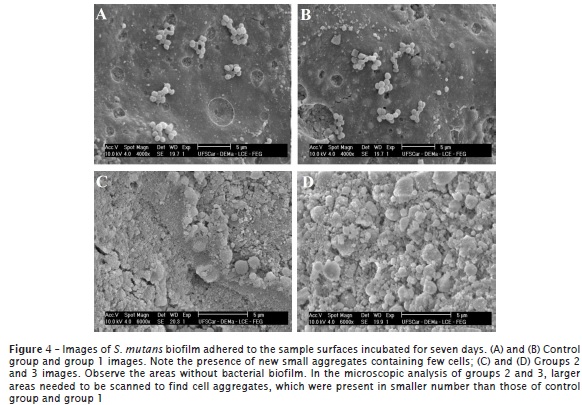
Figure 4 presents the images of the sample surfaces incubated after seven days. It was noted that the final phase of biofilm formation was taking place, that is, cell dispersion and adhesion to new sites, originating new cell aggregates. Both in control group and group 1, it was possible to verify the presence of these new, small aggregates with few cells and less complex than those registered in the images from four days of incubation. In groups 2 and 3, larger areas needed to be scanned to find those cell aggregates, which were seen in smaller number than those of control group and group 1.


Discussion
The result of this study indicates that the antibacterial activity of silver nanoparticles continued even after the mixture with resin composite, which was also seen by previous studies 1,5. Moreover, morphologic differences were seen in S. mutans biofilms adhered to the surfaces of silver-modified resins in relation to the biofilms formed on the surface of the conventional resin. To discuss this difference, it is important to consider that the biofilm development involves some phenomena, which have been divided didactically by many authors into formation stages, as follows 14:
• Initial adhesion to the surface; firstly reversibly, then, irreversibly;
• Formation of cell aggregates (or microcolonies), involved by exopolysaccharide;
• Growth and organization of the aggregates by forming internal channels for liquid and nutrient circulation, also involved by plentiful exopolysaccharide, characterizing the mature biofilm;
• Releasing of the cells of the mature biofilm, which colonized new sites, forming new biofilms, characterizing the final stage (dispersion or disaggregation).
Considering this formation process, it can be concluded that any substance acting on preventing the initial adhesion of the microorganisms or on any other biofilm development stage would be useful to control biofilm growth on surfaces and reduce the diseases caused by them. During the maturation stage, the biofilm thickness and complexity increase due to cell multiplication: structures containing large amounts of non-cellular material are formed, including exopolysaccharide and channels for nutrient circulation 9,14. The mature biofilm arrangement was only seen on the surface of the conventional resin composite.
Biofilm growth is limited by the nutrient availability that comes to the cells inside the structures, and by factors as pH, oxygen diffusion, carbon sources, and osmolarity. When the biofilm reaches some critical thickness, the most external layers release cells that colonize new surfaces, organizing new biofilms. Accordingly, it is possible to deduce that mature arrangements were seen in the images of four days of incubation on the surface of the conventional resin and then new small cell groups were found in the images of seven days of incubation. Thus, the biofilm was already mature at four days of incubation and following, the stage of cell dispersion and biofilm disaggregation took place on the surface of the conventional resin. Conversely, the typical biofilm formation did not take place on the samples of resin modified by silver nanoparticles, at all tested concentrations. Through microscopic images, none concentration displayed the stages of biofilm growth and mature biofilm arrangement.
The result of the cell counting was compatible with that of the morphologic analysis. At four and seven days of incubation, all groups of modified resin (groups 1, 2, and 3) showed number of viable cells statistically smaller than that of control group. This demonstrated that in the modified resin, the number of cells recovered from the biofilms was smaller over time together with the fact of the atypical evolution of the biofilm formation stages.
Father studies are necessary to verify if the inclusion of silver nanoparticles alters the physical properties of the resin composite, is toxic to the human being, and is released to the saliva. Some studies have already concluded that silver nanoparticles were not toxic to both animal and human cells at the concentrations used in biomaterials 20,27,29. It is important to continue the studies on resin composites modified by silver nanoparticles aiming to find a restorative material less favorable to bacterial adhesion and biofilm growth, to prevent secondary caries, gingivitis, and periodontal disease together with adequate oral hygiene.
Conclusion
The number of bacterial cells adhered on the surfaces of resin composite modified by silver nanoparticles at concentrations of 0.3 and 0.6%, (wt.) were statistically smaller than that on the conventional resin composite and on resin composite modified by silver nanoparticles at concentration of 0.1%, at the three tested incubation periods (1 day, 4 days, and 7 days). The microscopic images of the resin composites modified by silver nanoparticles demonstrated an atypical evolution of the cell arrangements during the biofilm formation process in comparison to the conventional resin composite. In conclusion, the antibacterial activity of silver nanoparticles continued to take place even after the mixture with the resin composite, resulting in biofilm growth control on the surface.
References
1. Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009 Feb;25(2):206-13. [ Links ]
2. Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010 Nov;89(11):1175-6.
3. Aykent F, Yodem I, Ozyesil AG, Gunal SK, Avunduk MC, Ozkan S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. Prosthet Dent. 2010 Apr;103(4):221-7.
4. Buergers R, Schneider-Brachert W, Hahnel S, Rosentritt M, Handel G. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent Mater. 2009 Feb;25(2):269-75.
5. Bürgers R, Eidt A, Frankenberg R, Rosentritt M, Schweiki H, Handel G et al. The ant-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch Oral Biol. 2009 Jun;54(6):595-601.
6. Catalan A, Herrera R, Martinez A. Denture plaque and palatal mucosa in denture stomatitis: scanning eléctron microscopic and microbiologic study. J Prosthet Dent. 1987 May;57(5):581-6.
7. Creanor SL. Fluoride uptake and release characteristics of glass ionomer cements. Caries Res. 1994 May;28(5):322-8.
8. Damm C, Münstedt H, Rosch A. Long-term antimicrobial polyamide/silver-nanocomposites. J Mater Sci. 2007 Nov;42(15):6067-73.
9. Dauqul M, Maciejewska M, Jaskiewicz M, Karafova A, Kamysz W. Antimicrobial peptides as potencial tool to fight bacterial biofilm. Acta Pol Pharm. 2014 Jan;71(1):39-47.
10. Davey ME, O'toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000 Dec;64(4):847-67.
11. De Muynck W, De Belie N, Verstraete W. Antimicrobial mortar surfaces for the improvement of hygienic conditions. J Appl Microbiol. 2010 Jan;108(1):62-72.
12. Dezelic T, Guggenheim B, Schmidlin PR. Multi-species biofilm formation on dental materials and an adhesive patch. Oral Health Prev Dent. 2009 Jan;7(1):47-53.
13. Elsaka SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: influence on physical and antibacterial properties. J Dent. 2011 Sep;39(9):589-98.
14. Ghigo JM. Are there biofilm-specific physiological pathways beyond a reasonable doubt? Res in Microbiol. 2003 Jan;154(1):1-8.
15. Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater. 2009 Jan; 28(1):11-9.
16. Kim J, Pitts B, Stewart PS, Camper A, Yoon J. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrob Agents Ch. 2008 Apr;52(4):1446-53.
17. Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013 Jun;11(6):371-84.
18. Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008 Nov;42(18):4591-602.
19. Lim BS, Lee HJ, Lee JW, Ahn SJ. Quantitative analysis of adhesion of cariogenic streptococci to orthodontic raw materials. Am J Orthod Dentofacial Orthop. 2008 Jun;133(6):882-8.
20. Panacek A, Kolar M, Vecerova R, Prucek R, Soukupova J, Krystof V. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009 Oct;30(31):6333-40.
21. Perdigão J, Ritter AV. Adesão aos tecidos dentários. In: Baratieri LN. Odontologia restauradora: fundamentos e possibilidades. 8 ed. São Paulo: Santos; 2001. Cap. 4. p. 85-128.
22. Philips RW. Resinas para restauração. In: Anusavice KJ. Materiais dentários. 10. ed. Rio de Janeiro: Guanabara; 1998. Cap. 12. p. 161-77.
23. Reddy MP, Venugopal A, Subrahmanyam M. Hydroxyapatite-supported Ag–TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007 Jan;41(2):379-86.
24. Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004 Apr;2(1):3-6.
25. Sanders BJ, Gregory RL, Moore K, Avery DR. Antibacterial and physical properties of resin modified glass-ionomers combined with chlohexidine. J Oral Rehab 2002 Jun; 29(6):553-8.
26. Sanpui P, Murugadoss A, Durga PV, Ghosh S, Chathopadhyay A. The antibacterial properties of a novel chitosan Ag-nanoparticle composite. Int J Food Microbiol. 2008 May;124(2):142-6.
27. Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD et al. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004 Nov;4(10):1881-7.
28. ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006 Sep;94(1):1-9.
29. Yudovin-Farber I, Beyth N, Nyska A, Weiss EI, Golenser J, Domb AJ. Surface characterization and biocompatibility of restorative resin containing nanoparticles. Biomacromolecules. 2008 Nov;9(11):3044-50.
30. Wolf HF, Hassel TM. Manual de Periodontia: fundamentos, diagnóstico, prevenção e tratamento. São Paulo: Artmed; 2008. p. 51-66.
 Corresponding author:
Corresponding author:
Patrícia Bolzan Agnelli das Neves
Universidade Federal de São Carlos – Departamento de Morfologia e Patologia
Rodovia Washington Luís, km 235, Caixa Postal 676
CEP 13565-905 – São Carlos – SP – Brasil
E-mail: patricia_bolzan@yahoo.com.br
Received for publication: June 9, 2014
Accepted for publication: June 26, 2014













