Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.12 no.2 Joinville Abr./Jun. 2015
Original Research Article
In vitro effectiveness evaluation of different substances in decontamination of intracanal metallic post
Humberto Ramah Menezes de MatosI; Ana Karine Duarte MaiaII; Cinthya Braga Alves TeixeiraII; Fábio de Almeida GomesIII; Claudio Maniglia-FerreiraIII; Márcia Maria de Negreiros Pinto RochaIV
I Department of Endodontics, School of Dentistry of Piracicaba, Unicamp – Piracicaba – SP – Brazil
II School of Dentistry, University of Fortaleza – Fortaleza – CE – Brazil
III Department of Endodontics, University of Fortaleza – Fortaleza – CE – Brazil
IV Department of Microbiology, University of Fortaleza – Fortaleza – CE – Brazil
ABSTRACT
Introduction: The intracanal metallic post, for many years, was the most used intracanal retainer and the best choice to restore endodontically treated teeth, showing until today high rates of success scientifically proven, good adaptation at the configuration of the root canal and resistance. Objective: The purpose of this study was to evaluate the effectiveness of some chemical substances in the decontamination of these intracanal metallic posts. Material and methods: Twenty intracanal metallic posts were divided into 6 experimental groups and 1 control group with 3 specimens each. The groups were divided into G1 (apple cider vinegar), G2 (0.12% chlorhexidine liquid), G3 (2% chlorhexidine gel), G4 (70° ethyl alcohol), G5 (2.5% sodium hypochlorite), and G6 (2% glutaraldehyde). For the control group was used saline solution. Each intracanal metallic post was submerged in your respective substance for 3 minutes and subjected to a smear dried sterile gauze. Immediately after this procedure the specimens were individually placed into tubes containing culture medium broth Brain Heart Infusion (BHI). The set of tubes containing the intracanal metallic post submerged in BHI were taken to the dry out machine and kept there at 37ºC for 48 hours. Results: The tubes that showed turbidity of BHI broth were considered positive, or contaminated. Conclusion: The methodology used in this study showed that all the disinfectants substances utilized were effective in decontaminating of the metallic post.
Keywords: chemical substances; dental prosthesis; decontamination.
Introduction
Many carious teeth can be conservatively restored with amalgam fillings or composite resin. However, when the endodontically treated teeth are extensively destroyed, many cases need for functional rehabilitation of an intracanal retainer or metallic post, whose primary function is to provide retention to the prosthetic crown or restorative material 4.
The intracanal metallic post (IMP) can be made in the office for direct or indirect techniques. In direct technique a modeling of root canal is made, and the indirect technique comprises the molding of root canal. Regardless of the manufacture technique, the IMP is fused at the dental lab, using casting techniques, and then sent to the office, where will be cemented. Thus, these materials come in contact with f luids such as saliva and / or blood, and therefore can be contaminated between the office and laboratory prosthesis.
Disinfection is an attempt to eliminate, usually by chemical means, pathogenic microorganisms from inanimate objects. It differs from the sterilization process because it is not an absolute procedure, or deletes only the vegetative and non-sporulated microorganisms. For this reason, whenever possible, one should make the sterilization 12.
To perform the surface disinfection, various chemical agents can be used. The initial step in the disinfection process incurs the knowledge of each of these products in their main aspects as its mechanism of action on microorganisms, toxicity and deleterious action handler for the material to be disinfected 10, 20.
This study aimed to evaluate the effect of 6 substances, 2.5% Sodium Hypochlorite (chlorinated soda, São Caetano do Sul, São Paulo, Brazil), 2% chlorhexidine gel (Chlorhexidine gel, Maringa , Paraná, Brazil), 0.12% chlorhexidine solution (0.12% chlorhexidine, Fortaleza, Ceará, Brazil), 70° ethanol (alcohol Santa Cruz, Guarulhos, São Paulo, Brazil), 2% glutaraldehyde (Glutacin 28, Serrana, São Paulo, Brazil), apple cider vinegar solution (vinegar fruit, Galvão Paulista, Pernambuco, Brazil) in the decontamination of IMP for 3 minutes.
Material and methods
This was an observational laboratory study, we aimed to evaluate the power of decontamination of 6 different chemicals means. Twenty-one IMP manufactured in the dental lab from the mold made in stock tooth and reproduction of the same in CAD -CAM (3M ESPE), resulting in 21 identical metallic post in all its dimensions. These twentyone IMPs were divided into 6 experimental groups (n = 3) (figure 1).
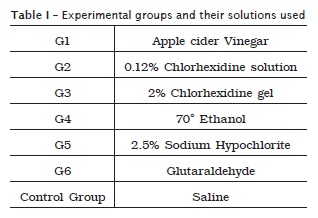
The experimental groups were div ided according to the product used for trying the decontamination of IMP: G1 represented by apple cider vinegar; G2 0.12% chlorhexidine solution; G3 2% chlorhexidine gel; G4 70° ethanol; G5 2.5% sodium hypochlorite; and G6 glutaraldehyde. A control group was used with saline for comparison. Each group had three IMPs and each was individually immersed in disinfectant substance for 3 minutes.
The specimens were then removed from the tested substances with the assistance of sterile forceps and then drying was carried out through sterile gauze swabs thereof, subsequently transferring the MFN was performed to test tubes containing 5 ml of Brain Heart Infusion (BHI, Difco Laboratories, Detroit, MI, USA). The whole experiment was performed using aseptic technique of sowing (table I).
All handling was performed using sterile gloves exchanged always at the end of manipulation of every IMP. Each tube contained only one IMP.
The tubes containing the IMP were incubated in a bacteriological incubator with controlled temperature of 37°C for 48 hours (figure 2). At the end of this period, the evaluation of the presence or absence of clouding of the culture medium (BHI) was carried out. In cases where there was the finding of the cloudy culture medium, we concluded that there was inefficiency in the IMP decontamination method. Since in those cases presenting the culture medium with no cloudy, we concluded that the method of decontamination was efficient.

Results
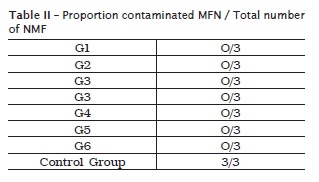
Table II shows the results of decontamination obtained with the products used. All products showed effectiveness in decontaminating IMP, since the control group (saline) showed clouding of BHI culture after 48h, suggesting the absence of action of this product against the bacteria found (figure 3).
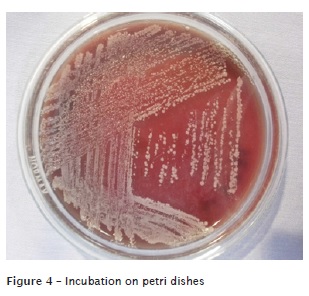
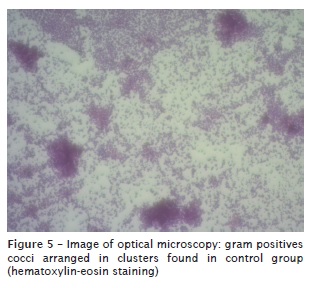
Cultures of bacteria present in the control group were performed on blood agar and lasted for 48 hours of incubation (figure 4). Catalase and coagulase testing was conducted in these colonies and the same was visualized in an optical microscope. It was determined through these tests that the bacterial species present in the control group was Staphylococcus sp. coagulase-negative. The visualization of the slides was performed with an optical microscope and observed, the entire arrangement in clusters (figure 5).

Discussion
In this study we evaluated the efficacy of disinfection of IMP collected in the dental lab, used in the disciplines of the University of Fortaleza (UNIFOR). The samples were submerged for 3 minutes in 7 substances daily use in dental offices (2.5% sodium hypochlorite, 2% chlorhexidine gel, and 0.2% chlorhexidine solution, 70% ethanol, 2% glutaraldehyde, and saline) and the vinegar used for cleaning vegetables. Microbiological evaluation of the bacterial growth was through two tests growth in liquid culture medium, blood agar, and BHI broth, which allow the growth of wide variety of microorganisms, such as Streptococcus pyogenes, Staphylococcus aureus, pneumococcus, meningococcus, Enterobacteriaceae, yeast and fungi.
It is known that dental laboratories are highly contaminated atmospheres, where large amounts of circulating pathogenic microorganisms, constituting a high risk for technicians, dentists and patients 2, 16, 19, 20, 22, 24, 25, 26.
Sodium hypochlorite is used extensively in endodontics, as irrigant agent for root canals, to contribute to the cleaning and sanitizing. It has low cost and easy handling, including through the dilution of sanitary water sold in supermarkets. Nevertheless, it has some disadvantages such as sensitivity to light and temperature, which demands care in storage so it does not lose its effectiveness. The findings of this study agreed with the literature reviewed, since it proved the antimicrobial activity of this solution in all specimens disinfected with it 15, 17.
Glutaraldehyde is a substance commonly used for disinfection of heat-sensitive materials and instruments. According to the Brazilian Health Surveillance Agency 1, it requires 20 minutes of exposure to result in a high-level disinfection, but the time of 3 minutes was efficient as demonstrated in our research.
Ethanol at 70° has activity against bacteria in the vegetative form, enveloped viruses (e.g.: viruses causing influenza, hepatitis B and C, and AIDS), mycobacteria and fungi. It does not present action against spores and non-enveloped viruses (e.g.: hepatitis A virus and rhinovirus), characterizing itself as a disinfectant and antiseptic, but without sterilizing property.
Vinegar is a product of fermentation and is used in the cleaning of salads, but many are not aware of its disinfectant action, which was proven in this study.
The time period used for this study was 3 minutes. The Brazilian Ministry of Health (2000) 6 recommends disinfecting prostheses containing metal in its structure, with immersion in sodium hypochlorite or glutaraldehyde for 10 minutes. ANVISA 1 suggests a time of 10 minutes of immersion in glutaraldehyde for common disinfection and 20 minutes for high-level disinfection. This study found all samples submitted to three minutes of submersion were disinfected.
There are not studies correlating the cementing of contaminated IMP with endodontic treatment failure and the need for retreatment. However, it can be assumed that a contaminated metallic post into the root canal, combined with a poor filling, it would be a possible cause of persistent pathologies 9, 11, 18, 22.
The risk of the occurrence of cross-contamination and the likely risk of endodontic manipulation and cementation of contaminated IMP justify the search for an effective disinfection protocol to eliminate the microorganisms present in these.
Ideally, the procedures to control cross infection by disinfecting sent and received prosthetic work should be performed routinely, both by dental technicians, as well as by dentists. However, both the literature, as the results obtained in this study show that these protocols are generally not adopted 2, 3, 5, 7, 8, 13, 14, 16, 19, 21, 24-26.
Conclusion
Based on the results of our study, it can be concluded that all used substances were effective for metallic root canal post disinfection and therefore it can be routinely used in dental practice.
References
1. Agência Nacional de Vigilância Sanitária. Informe técnico n.º 04/07 – Glutaraldeído em estabelecimentos de atenção à saúde. 2007.
2. Agost inho AM, Miyoshi PR, Gnoat to N, Paranhos HFO, Figueiredo LC, Salvador SL. Crosscontamination in the dental laboratory through the polishing procedure of complete dentures. Braz Dent J. 2004;15(2):138-43.
3. Al-Dwairi ZN. Infection control procedures in commercial dental laboratories in Jordan. JDE. 2007;71(9):1223-7.
4. Bassols JMC. Reconstrucción de dientes endodonciados. 1ª ed. Madri: Pues S. L.; 2005.
5. Bôas MV, Quirino MRS. Controle de infecção cruzada: laboratório de prótese versus consultório odontológico. R Bras Bioci. 2002;8(1):103-8.
6. Brasil. Ministério da Saúde. Secretaria de Políticas de Saúde, Coordenação Nacional DST e Aids. Controle de infecção e a prática odontológica em tempos de Aids: manual de condutas. Brasília; 2000.
7. Campanha NH, Pavarina AC, Vergani CE, Machado AL, Giampaolo ET. Cross-infection control policy adopted by dental technicians. Rev Odontol UNESP. 2004;33(4):195-201.
8. Cotrim LEF, Santos EM, Jorge AC. Procedimentos de biossegurança realizados por cirurgiões-dentistas e laboratórios durante a confecção de próteses dentárias. Rev Odontol UNESP. 2001;30(2):233-44.
9. Estrela C, Estrela CRA, Barbin EL, Spanó JCE, Marchesan MA, Pécora JD. Mechanism of action of sodium hypochlorite. Braz Dent J. 2002;13(2):113-7.
10. Jorge AOC. Princípios de biossegurança em Odontologia. R Bras Bioci. 2002;8(1):7-17.
11. Leonardo MR, Silva LAB, André RFC, Bonifácio KC, Ito IY. Evaluation of the sterility of absorbent paper points. Braz Dent J. 1997;2(2):31-2.
12. Leonardo MR. Endodontia: tratamento de canais radiculares: princípios técnicos e biológicos. São Paulo: Artes Médicas; 2008.
13. Majewski M, Koga-Ito Y, Junqueira JC, Jorge AOC. Avaliação das condutas de biossegurança aplicadas em laboratórios de prótese dentária. Rev Bras Bioc. 2004;10(3):161-6.
14. Mathias SA, Mathias AL, Guandalini SL. Detecção de pontos críticos no controle de infecção em laboratórios de prótese. JBC. 1998;2(8):51-7.
15. Mcgowan MJ, Shimoda LM, Woolsey GD. Effects of sodium hypochlorite on denture base metals during immersion for short-term sterilization. J Prosthet Dent. 1988;60(2):212-8.
16. Moriello KA, Hondzo H. Efficacy of disinfectants containing accelerated hydrogen peroxide against conidial arthrospores and isolated infective spores of Microsporum canis and Trichophyton sp. Vet Dermatol. 2014 Jun;25(3):191-4.
17. Pavarina AC, Pizzolitto AC, Machado AL, Vergani CE, Giampaolo ET. An infection control protocol: effectiveness of immersion solutions to reduce the microbial growth on dental prostheses. J Oral Rehabil. 2003;30(5):532-6.
18. Pinheiro ET. Investigação de bactérias associadas ao insucesso do tratamento endodôntico [dissertação – Mestrado em Odontologia]. Piracicaba: Universidade Estadual de Campinas; 2000.
19. Powell GL, Runnells RD, Saxon BA, Whisenant BK. The presence and identification of organisms transmitted to dental laboratories. J Prosthet Dent. 1990;64(2):235-7.
20. Silva EMS. Avaliação da infiltração marginal apical da obturação de canais radiculares. Efeito da técnica de obturação [dissertação de Mestrado]. Camaragibe: Universidade de Pernambuco. Faculdade de Odontologia de Pernambuco; 1992.
21. Schroeder KM, Jacobs RA, Guite C, Gassner K, Anderson B, Donnelly MJ. Use of a chlorhexidineimpregnated patch does not decrease the incidence of bacteria l coloni zat ion of femora l ner ve catheters: a randomized trial. Can J Anaesth. 2012;59(10):950-7.
22. Sundqvist G1, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative retreatment. Oral Sur Oral Med Oral Pathol Oral Radiol Endod. 1998;85(1):86-93.
23. Verran J, Kossar S, McCord JF. Microbiological study of selected risk areas in dental technology laboratories. J Dent. 1996;24(1-2):77-80.
24. Vojdani M, Zibaei M. Frequency of bacteria and fungi isolated from pumice in dental laboratories. J Res Health Sci. 2006;6(1):33-8.
25. Wakefield CW. Laboratory contamination of dental prostheses. J Prosthet Dent. 1980;44(2):143-6.
26. Wi l l iams HN, Fa lkler WA, Hasler JF, Libonati JP. The recovery and significance of nonoral opportunistic pathogenic bacteria in dental laboratory pumice. J Prosthet Dent. 1985;54(5):725-30.
 Correspondence:
Correspondence:
Humberto Ramah Menezes de Matos
Rua Bill Cartaxo, 885 – casa 16 – Água-Fria
CEP 60833-185 – Fortaleza – CE – Brasil
E-mail: beto_meneses@hotmail.com
Received for publication: November 11, 2014
Accepted: March 6, 2015













