Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.12 no.3 Joinville Jul./Set. 2015
ORIGINAL RESEARCH ARTICLE
Validation of pH cycling model to induce artificial carious lesions in bovine dentin
Ana Caroline FumesI; Raquel Assed Bezerra da SilvaI; Daniele Lucca LongoI; Andiara De RossiI; Mônica Campos SerraII
I Department of Pediatric Clinics, Ribeirão Preto Dental School, University of Sao Paulo – Ribeirão Preto – SP – Brazil
II Department of Restorative Dentistry, Ribeirão Preto Dental School, University of Sao Paulo – Ribeirão Preto – SP – Brazil
ABSTRACT
Introduction and objective:The purpose of the study was to evaluate different pH cycling protocols on the induction of artificial carious lesions in bovine dentin, since the most appropriate protocol to be applied is still not fully established. Material and methods: Fragments of bovine dentin (4 x 4 x 2 mm) were embedded in resin, polished and 7 mm² of each fragment was isolated with wax. The specimens were divided into three groups (A, B, C) according to the time of immersion in the demineralizing solution (1.5 ml). Group A – 15 minutes; Group B – 30 minutes; Group C – 60 minutes and subsequently immersed for 22 hours in a remineralizing solution (1.5 ml). Microhardness measurements were conducted initially, daily and after each pH cycling for 4 days. The Split-plot design (ANOVA) was applied. Results: There was a significant interaction between time and cariogenic challenge (p<0.0001). Bonferroni comparisons were executed to identify the differences over the cariogenic challenge, showing that increasing the immersion time in demineralizing solution for each pH cycling assessed, the cariogenic challenge aggressiveness increased (A <B <C). Also, for each protocol tested there was a significant decrease in the hardness in the cariogenic challenge over the time. Conclusion: The three models tested proved to be viable, regardless of the time of cariogenic challenge that was applied.
Keywords: dental caries, tooth remineralization, tooth demineralization.
Introduction
Dental caries are a result of changes in pH caused by acids driven by the biofilm (in contact with mineralized tissues), reducing the mineral content of these tissues 16,31. Although the process of formation of caries is currently better understood, there are still many details to be investigated 7,8.
Methods and protocols have been proposed and used for the induction of artificial caries 1,5,11,19,24,27,28. Considering the limitations of in vitro studies, the protocols that use pH cycling are those that most resemble the natural process, simulating events of demineralization and remineralization, as it happens clinically 29.
Models using pH cycling for the development of caries have been proposed in bovine dentin 5,11-14,19,21,25,30.
The bovine substrate presents a more uniform composition, which enables a smaller variability in experimental response 18. Furthermore, bovine dentin has similar characteristics to human dentin, in terms of diameter and number of dentinal tubules 6,9,26. In addition, several studies have used these teeth as a substrate due to the ethical aspects and also because they are more easily obtained and manipulated 6,11-14,19,24,26,30.
However, there is still no standardized protocol for the induction of artificial caries using pH cycling in bovine substrate.
The objective of this study was to evaluate the protocols of induction of caries in vitro, through the use of pH cycling on bovine teeth in order to contribute to the standardization of studies and a better understanding of the lesions progression.
Material and methods
Experimental design
The object of the study was a model that induces caries performed in three different immersion times: 15 minutes of demineralization (DE) and 22 hours of remineralization (RE); 30 minutes of DE and 22 hours of RE, and 60 minutes of DE and 22 hours of RE. Forty-five experimental fragments of bovine dentin (n = 15) were used. The used variable was the surface microhardness test (Knoop).
Preparation of the specimens
Bovine teeth were sectioned precisely (Isomet 1000, Buehler), in cementoenamel junction, to separate the coronal from the root portion. New sections were performed, yielding ten dental fragments measuring 4 x 4 x 2 mm. Then these fragments were embedded in polyester resin (Milflex), exposing only the external face.
After the inclusion in resin, the specimens were then flattened and polished (β Phonix – Buehler) with a decreasing granulation sandpaper of Al2O3 (400, 600 and 1200 grit) and an abrasive alumina, under water cooling (4). This was performed until the exposition of the dentin.
Subsequently, the specimens were washed with deionized water and subjected to ultrasound for 10 minutes in deionized water and then identified and stored individually in containers with relative humidity of 37ºC.
Standardization in the area of lesion induction
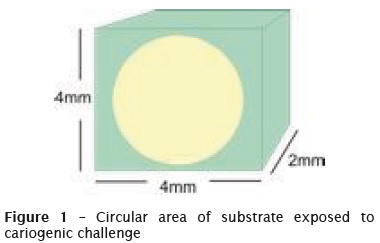
On the surface of each specimen of dentin, a circular area about 7 mm² was left exposed (figure 1). This area was isolated with a standard nail polish, and the exposed dentin surface left to contact with the DE and RE solutions for the dynamic induction of the carious lesions.
Readings of initial microhardness
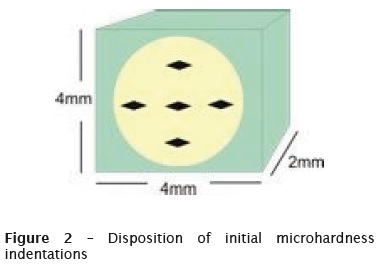
The specimens initially prepared, were assessed for their initial surface microhardness. To this purpose, HMV-2 microdurometer and a Knoop indenter with a load of 10 grams for 10 seconds were used in the dentin (defined in the preliminary tests). There were five indentations in each tested fragment (figure 2) and in a total of 70 specimens, 45 were selected.
pH cycling
The artificial caries in dentin were induced by a dynamic model of demineralization and remineralization, similar to that described by Hara et al. 11. Other studies that applied pH cycling, inducing lesions in both enamel 2 and dentin 12,14, added fluorine to the solutions 2,12,14, and limited the time of solution in DE to decrease the aggressiveness of the cariogenic challenge.
The solutions applied were: DE solution (1.4 mM Ca, 0.9 mM P, 0.03 ppm F, 0.05 M acetate buffer, pH 5.0); RE solution (1.5 mM Ca, 0.9 mM P, 0.05 ppm F, 0.1 M Tris buffer, pH 7.0).
Forty-five specimens were then divided into three groups (A, B, C) (n = 15) according the immersion time in DE solution (1.5 ml): group A – 15 minutes; group B – 30 minutes; group C – 60 minutes and afterwards, immersed for 22 hours in RE solution (1.5 ml). Microhardness measurements were conducted initially, daily and after each pH cycling for 4 days.
Statistical analysis
For the statistical measuring, the average values of microhardness obtained initially and after each pH cycling, were considered. After checking the homoscedasticity and normality, the Split-Plot design (ANOVA) was used - because there where repeated measures - in the evaluation of the effect of cariogenic challenge factors and in the number of pH cycles, as recommended by Montgomery20. Since the interaction between the factors was positive, Bonferroni comparisons were performed to identify differences over the cariogenic challenges.
Results
The split-plot design (ANOVA) was performed, and the adjustment of the (R2) model was 0.8884, indicating the adequacy of the mathematical model to analyze the data. There was a significant interaction between the time-cariogenic challenge (p <0.0001).
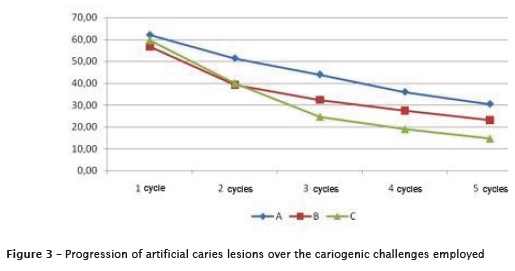
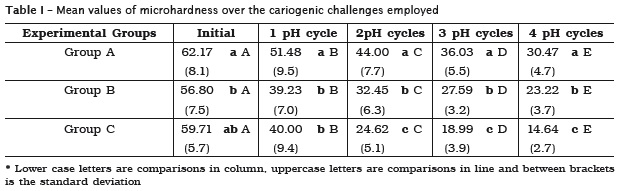
The increase of immersion time in DE solution for each pH cycle, increased the aggressiveness of the cariogenic challenge (A <B <C). The microhardness values of the dentin showed a significant decrease along the cariogenic challenge (figure 3). Table I illustrates data concerning the decrease in microhardness values.
Discussion
The result of dental caries is determined by a dynamic balance between pathological factors that lead to demineralization and protective factors that promote remineralization 8,17. Among the existing models for the induction of caries, all have advantages and limitations. However, the use of pH cycling is considered the closest and more 29 dynamic process of formation of carious lesions (alternating periods of demineralization and remineralization) 7,8,29.
Thus, based on a model of pH cycling, described by Featherstone et al. (1986) and modified by Hara et al. 11, so that the microhardness readings could be made in dentin surface, over the time of the cariogenic challenge, modifications to the model had to be performed 3. The volume and time of immersion in demineralizing solution were decreased. Also were added small concentrations of fluorine (0.03 ppm in demineralizing solution and 0.05 in the remineralizing solution), these concentrations based on the model described by Argenta et al. 2.
Considering fluoride, the key to the control of dental caries, it is known that it can act to inhibit demineralization, inhibit bacterial enzymes and enhance remineralization 7. The addition of fluoride and other changes were to contain the aggressive agent promoted by the cariogenic challenge and thereby enabling microhardness measurements daily. It is considered that the evaluations of the changes, in the values of the surface microhardness of the dental substrates, are an extremely important factor in relation to demineralization and remineralization processes, since the main interactions between the dental tissues and oral environment occur at the surface layer 2.
In this in vitro study, the methodology applied for the induction of artificial caries lesions allowed the induction of a demineralized dentin in both substrates.
Unlike the enamel, dentin presents a lower mineral content – is formed by inorganic (70%), organic material (20%) and water (10%) components (23) – and the used model as an absence of organic compounds in the remineralizing solution. The formation of caries clinically, can extend for weeks, months or years, given the biological responses that may occur as the formation of reactionary dentin 32.
In contrast to the induction of artificial caries, which is usually accomplished in few hours. Another aspect to be considered is the fact that some authors criticize the use of microhardness to evaluate changes in the dentin. Guided by a shrinkage of the indentation that needs to be done 32, otherwise the results are incorrect. However, in the study the microhardness data were obtained from specimens that were not hydrated and the values of hardness were measured immediately after the indentation was performed. Thereby avoiding the influence of the elastic deformation 15, and standardizing the conditions for all samples.
The results of this study showed that based on the application of a pH cycling model in dentin, the longer the solution of demineralization was applied, the faster the loss of mineral content, which may be observed by the decrease in microhardness values. The application of such methodology may be relevant to studies that suggest, for example, the evaluation of adhesive systems in dentin. It is known that the bond strength studies are usually conducted in healthy dentine, for convenience and comfort, but the truth is that clinically, the dentin adhesive system is not applied in a healthy substrate, but in an sclerotic and demineralized dentin 4,10,22, similar to the one obtained in this study. On the other hand, pH cycling model studies in enamel can also be performed, as showed after comparing the effects of 5 children's toothpastes (calcium phosphate, Pooneh, Biotin, Crest and Darougar) 17.
The knowledge obtained from this study can be used as a viable model for the evaluation and development of measures and materials that can help controlling the in vivo process. It can be concluded that all three models tested were shown to be viable in obtaining artificial carious lesions in bovine dentin, regardless of the time of cariogenic challenge applied.
Conflic of interest
All the authors state that they have no conflicts of interest.
References
1. Amaechi BT, Higham SM, Edgar WM. Factors affecting the development of carious lesions in bovine teeth in vitro. Arch Oral Biol. 1998 Aug;43(8):619-28. [ Links ]
2. Argenta RMO, Tabchoury CPM, Cury JA. A modified pH-cycling model to evaluate fluoride effect on enamel demineralization. Pesquisa Odontológica Brasileira. 2003;17:241-6.
3. Comar LP, Souza BM, Gracindo LF, Buzalaf MA, Magalhães AC. Impact of experimental nano-HAP pastes on bovine enamel and dentin submitted to a pH cycling model. Braz Dent J. 2013;24(3): 273-8.
4. Choi K, Oshida Y, Plat JA, Cochram MA, Matis BA, Yi K. Microtensile bond strength of glass ionomer cements to artificially created carious dentin. Operative Dentistry. 2006;31:590-7.
5. de Menezes M, Turssi CP, Faraoni-Romano JJ, Serra MC. Susceptibility of bleached enamel and root dentin to artificially formed caries-like lesions. Am J Dent. 2007;20:173-6.
6. Dutra-Correa M, Anauate-Netto C, Arana-Chavez VE. Density and diameter of dentinal tubules in eatched and non-eatched bovine dentine examined by scanning electron microscopy. Arch Oral Biol. 2007;850-5.
7. Featherstone JD. Caries detection and prevention with laser energy. Dent Clin North Am. 2000 Oct;44(4):955-69.
8. Featherstone JD. The continuum of dental caries evidence for a dynamic disease process. J Dent Res. 2004;83:39-42.
9. Garberoglio R, Brännström M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21:355-62.
10. Marshall Jr GW, Marshall SJ, Kinneyt JH, Balooch Mehdi. The dentin substrate: structure and properties related to bonding. Journal of Dentistry. 1997;25:441-58.
11. Hara AT, Queiroz CS, Freitas PM, Giannini M, Serra MC, Cury JA. Fluoride release and secondary caries inhibition by adhesive systems on root dentine. Euro J Oral Sci. 2005;113:245-50.
12. Hara AT, Queiroz CS, Giannini M, Cury JA, Serra MC. Influence of the mineral content and morphological pattern of artificial root caries lesion on composite resin bond strength. Eur J Oral Sci. 2004;112:67-72.
13. Hara AT, Queiroz CS, Paes Leme AF, Serra MC, Cury JA. Caries progression and inhibition in human and bovine root dentine in situ. Caries Res. 2003;37:399-44.
14. Hara AT, Magalhães CS, Serra MC, Rodrigues Jr AL. Cariostatic effect of fluoride-containing restorative systems associated with dentifrices on root dentin. Journal of Dentistry. 2002;30: 205-12.
15. Herkströter FM, Witjes M, Ruben J, Arends J. Time dependency of microhardness indentations in human and bovine dentine compared with human enamel. Caries Res. 1989;23:342-4.
16. Kidd EAM. Fejerskov O. What constitutes dental caries? Histopatology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res. 2004;83 (Spec Iss C):C35-8.
17. Malekafzali B, Ekrami M, Mirfasihi A, Abdolazimi Z. Remineralizing effect of child formula dentifrices on artificial enamel caries using a pH cycling model. J Dent (Tehran). 2015 Jan;12(1):11-7.
18. Mellberg JR. Hard-tissue substrates for evaluation of cariogenic and anti-cariogenic activity in situ. J Dent Res. 1992 Apr;71:913-9.
19. Molina GF, Costa de Almeida GR, de Souza Guerra C, Cury JA, de Almeida AP, Barroso RC et al. Lead deposition in bovine enamel during a pH-cycling regimen simulating the caries process. Caries Res. 2011;45:469-74.
20. Montgomery DC. Design and analysis of experiments. 4th ed. 1997.
21. Mukai Y, ten Cate JM. Remineralization of advanced root dentin lesions in vitro. Caries Res. 2002;36:275-80.
22. Nakajima M, Sano H, Burrow MF, Tagami J, Yoshiyama M, Ebisu S et al. Tensile bond strength and SEM evaluation of caries-affected dentin using dentin adhesives. J Dent Res. 1995;74:1679-88.
23. Nanci A. Ten Cate's oral histolog: development, structure and function. 6th ed. 2003.
24. Queiroz CS, Hara AT, Paes Leme AF, Cury JA. pH-cycling models to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz Dent J. 2008;19:21-7.
25. Rodrigues E, Delbem AC, Pedrini D, Cavassan L. Enamel remineralization by fluoride-releasing materials: proposal of a pH-cycling model. Braz Dent J. 2010;21:446-51.
26. Schilke R, Lisson JA, Bauss O. Geurtsen W. Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Arch Oral Biol. 2000;45:355-61.
28. Serra MC, Cury JA. The in vitro effect of glassionomerr cement restoration on enamel subjected to a desmineralization and remineralization model. Quintessence Int. 1992;23:143-7.
29. ten Cate JM, Duijsters PPE. Aternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16:201-10.
30. ten Cate JM. In vitro studies on the effects of fluoride on de- and remineralization. Journal of Dental Research. 1990;69 (Spec Issues):614-9.
31. Turssi CP, Lima RQ, Faraoni-Romano JJ, Serra MC. Rehardening of caries-like lesions in root surfaces by saliva substitutes. Gerodontology. 2006;23:226-30.
32. Vyavhare S, Sharma DS, Kulkarni VK. Effect of three different pastes on remineralization of initial enamel lesion: an in vitro study. J Clin Pediatr Dent. 2015 Winter;39(2):149-60.
33. Wefel JS, Heilman JR, Jordan TH. Comparison of in vitro root caries models. Caries Res. 1995;29:204-9.
 Corresponding author:
Corresponding author:
Alvaro Cruz
Av. Francisco Javier Gamboa, n. 230, Col. Arcos Sur
CP 44150 – Guadalajara – Jalisco – México
E-mail: carolfumes02@hotmail.com
Received for publication: December 22, 2014
Accepted for publication: September 14, 2015













