Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.12 no.3 Joinville Jul./Set. 2015
ORIGINAL RESEARCH ARTICLE
Analysis of the time required for dissolving the pulp tissue according to different methods of sodium hypochlorite activation
Ana Luíza LeichtweisI; Tiago André Fontoura de MeloI; Gustavo Golgo KunertI
I Department of Dentistry, Center of Post-Graduation São Leopoldo Mandic/SP, unit Porto Alegre – Porto Alegre – RS – Brazil
ABSTRACT
Introduction and Objective:To analyze the time required to dissolute the pulp tissue under different methods of sodium hypochlorite activation. Material and methods: 30 bovine pulp fragments, with an approximate volume of 45 ± 5 mg were divided into three experimental groups (n = 10). In group 1, only the fragments were immersed in 15 ml of 2.5% sodium hypochlorite. In group 2, the irrigant was manually stirred with an endodontic instrument type K size 40. In group 3, we used the ultrasonic insert n.39, driven by ultrasound device Jet Sonic Total. The time required for total dissolution of bovine tissue was measured and recorded for statistical analysis. Results and Conclusion: According to analysis of variance (Anova), with p<0.05, Group 1 showed significantly higher dissolution time than Groups 2 and 3. Moreover, no difference between the use of ultrasound and manual activation of the irrigating solution was found.
Keywords: dental pulp; dissolution; ultrasound.
Introduction
While the mechanical action shapes the root canal, the chemical action acts to inactivate microorganisms and eliminate the organic and inorganic components present therein.
One of the most used and accept abl e solutions for endodontic irrigation is sodium hypochlorite. Hypochlorite properties are based on its concentration, temperature, and pH 18. This irrigating solution has low surface tension 3, antimicrobial action 19, and dissolution capacity of organic matter 14.
However, because of the root canal system present a complex anatomy, with the lateral canals, isthmuses, ramifications and apical delta, the efficiency of irrigation is compromised on the action of dissolving pulp tissue in dentin reentrances 15. Some studies suggest conducting a process of active irrigation in order to increase the effectiveness in cleaning the root canal system 1,5,8. According to Juchem et al. 10, the contact area of the solution can be increased by activation through either mechanical or hand technique.
Thus, this study aimed to analyze the dissolution capacity of the pulp tissue according to different methods for the activation of 2.5% sodium hypochlorite.
Material and methods
Tissue preparation
Fifteen bovine incisors were obta ined in Slaughterhouse of São Roque/Cerro Largo (RS). These teeth were immersed in distilled water and stored at a temperature of -20° C until use.
The teeth were defrosted at room temperature. Two longitudinal grooves were made with a doublesided diamond disc (KG Sorensen, Barueri, São Paulo, Brazil), on all the fullest extent of the tooth sample at the buccal and lingual surfaces, in order to enable tooth cleavage with size 7 spatula (Golgran, São Paulo, São Paulo, Brazil).
The pulp tissue was removed and washed with distilled water. Each tissue sample was divided into two fragments of similar size (45 ± 5 mg), resulting in a total of 30 parts of pulp tissue.
Tissue dissolution process
The bovine tissue fragments were divided and placed into individual clears plastic flasks and randomly divided into three groups (n = 10). The experiment of all groups was run at the same day.
In group 1, the pulp fragments were only immersed in 2.5% sodium hypochlorite solution at room temperature (Asfer – Chemical Industry Ltda., São Caetano do Sul, São Paulo, Brazil.).
In group 2, the tooth samples were immersed in 2.5% sodium hypochlorite solution and the solution was hand activate with size #40 K-type endodontic instrument (Dentsply Maillefer, Ballaigues, VD, Switzerland). This protocol was performed three times for 40 seconds (totalizing 120 seconds).
In group 3, we used the same mechanism of action of group 2, but with the ultrasonic insert #39 for agitation. The tip was employed with power of 30% and without water (Jet Sonic Total, Gnatus Medical Dental Equipment Ltda., Ribeirão Preto, São Paulo, Brazil). Time and the solution agitation cycle in group 3 was the same of group 2.
The amount of sodium hypochlorite solution in each flask was standardized at 15 ml. The time counting for dissolving tissue was started at the time of immersion of the bovine pulp fragment into the solution with the aid of a digital stopwatch.
The observation of complete tissue dissolution was conducted by a single examiner, without any knowledge on the objectives of the study. The time obtained was noted in a spreadsheet.
The maximum time set for the experiment was 2 hours. If the pulp tissue was not completely dissolved after that time the sample was considered unable to promote tissue dissolution.
Statistical analysis
The data were submit ted to ANOVA, by comparing the average percentage of dissolution in the three experimental groups. The significance level was set at 5% (P<.05).
Results
None group elapsed the maximum time set for the experiment.
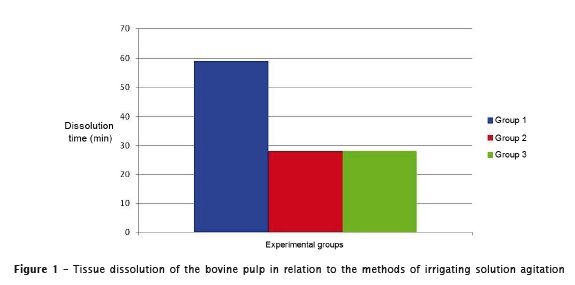
As shown in Figure 1, the pulp tissue in group 1 took approximately 60 minutes to be completely dissolved. In groups 2 and 3, the average time was 30 minutes. No statistical difference between was seen between the use of agitation with either endodontic or ultrasonic instrument.
Discussion
The use of irrigating solution during root canal treatment is justified by the fact that there are variations in internal anatomy of the root canal system that hinder the action of endodontic instruments around the dentin complex.
In this present study, we chose sodium hypochlorite because it is the solution most used in Endodontics and the most cited in the literature. According to Cobankara et al. 4 and Morgental et al. 13, hypochlorite has the power of tissue dissolution and an antimicrobial capacity exceeding the other irrigant solutions.
Based on the studies of Hand et al. 9 and Moorer and Wesselink 12, it is known that the tissue dissolution capacity caused by sodium hypochlorite solution is related to a number of factors, such as their concentration, volume and temperature.
Thus, caution was taken to standardize the study regarding to the volume of 15 ml of solution used 10 and the agitation time, both manual and mechanical (ultrasound) for three cycles of 40 seconds.
With regard to the use of bovine pulp, this has been successfully employed in the studies of Al-Jadaa et al. 2 and Macedo et al. 11 to assess the dissolution power of endodontic irrigants.
The result analysis showed that, regardless of the agitation method of the irrigant solution, the tissue dissolution time was shorter in the group which the pulp tissue remained only immersed into the solution. These results corroborate those from Stojicic et al. 17, who found that agitation promoted greater dissolution capacity.
The lack of statistically significant difference between the groups 2 and 03 (manual and ultrasonic) in the present study can be justified because one is unable to measure the similar relationship between manual and ultrasonic agitation.
One of the phenomena caused by the ultrasonic oscillation is called cavitation. 6. Cavitation is limited to a distance of less than 100 μm (0.0001 m) from the generating source. Thus, it is difficult to measure whether the power loss of this phenomenon influenced on the result of this study, because bovine pulp was immersed into sodium hypochlorite at a depth of 10 mm (0.01 m). According to the study of Stojicic et al. 17, probably only the effect caused by the phenomenon of acoustic micro stream might have occurred
Moreover, a greater difference occurred in the dissolution time, when the liquid remained static compared to the solution that had undergone agitation; which is in agreement with the study of Só et al. 16. The authors found that 2.5% hypochlorite solution completely dissolved samples in less than two hours probably because the movement of the solution causes a larger number of solid particles are in contact with the fluid by increasing the contact surface, since the contact area is a factor to consider in dissolution10. Guneser et al. 7 also found a higher tissue dissolution capacity with agitation of the irrigant solution.
Conclusion
According to the results, it can be concluded that:
• The process of agitation of 2.5% sodium hypochlorite solution showed a reduction in bovine pulp tissue dissolution time by 50% compared to no agitation;
• There was no statistical difference in tissue dissolution time in relation to the use of an endodontic instrument or an ultrasonic tip for agitation of sodium hypochlorite solution.
References
1. Al-Jadaa A, Paqué F, Attin T, Zehnder M. Acoustic hypochlorite activation in simulated curved canals. J Endod. 2009;35(10):1408-11. [ Links ]
2. Al-Jadaa A, Paqué F, Attin T, Zehnder M. Necrotic pulp tissue dissolution by passive ultrasonic irrigation in simulated accessory canals: impact of canal location and
angulation. Int Endod J. 2009;42(1):59-65. 3. Andersen M, Lund A, Andreasen JO, Andreasen FM. In vitro solubility of human pulp tissue in calcium hydroxide and sodium hypochlorite. Endod Dent Traumatol. 1992;8(3):104-8.
4. Cobankara FK, Ozkan HB, Terlemez A. Comparison of organic tissue dissolution capacities of sodium hypochlorite and chlorine dioxide. J Endod. 2010;36(2):272-4.
5. Deleu E, Meire MA, De Moor RJ. Efficacy of laser based irrigant activation methods in removing debris from simulated root canal irregularities. Laser Med Sci. 2015;30(2):831-5.
6. Grundling GL, Zechin JG, Jardim WM, de Oliveira SD, de Figueiredo JA. Effect of ultrasonics on Enterococcus faecalis biofilm in a bovine tooth model. J Endod. 2011;37(8):1128-33.
7. Guneser MB, Arslan D, Usumez A. Tissue dissolution ability of sodium hypochlorite activated by photon-initiated photoacoustic streaming technique. J Endod. 2015;41(5):729-32.
8. Haapasalo M, Wang Z, Shen Y, Curtis A, Patel P, Khakpour M. Tissue dissolution by a novel multisonic ultracleaning system and sodium hypochlorite. J Endod. 2014;40(8):1178-81.
9. Hand RE, Smith ML, Harrison JW. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod. 1978;4(2):60-4.
10. Juchem CB, Pereira GB, Soares RG, Irala LED, Salles AA, Limongi O. Avaliação da capacidade de dissolução de tecido pulpar bovino pelo ácido tricloroisocianúrico nas concentrações de 1%, 2%, 3% e 4% comparativamente ao hipoclorito de sódio 1%. RSBO. 2008;5(1):34-41.
11. Macedo RG, Wesselink PR, Zaccheo F, Fanali D, Van Der Sluis LW. Reaction rate of NaOCl in contact with bovine dentine: effect of activation, exposure time, concentration and pH. Int Endod J. 2010;43(12):1108-15.
12. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982;15(4):187-96.
13. Morgental RD, Singh A, Sappal H, Kopper PM, Vier-Pelisser FV, Peters OA. Dentin inhibits the antibacterial effect of new and conventional endodontic irrigants. J Endod. 2013;39(3): 406-10.
14. Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30(11):785-7.
15. Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obtured in the presence or absence of detectable microorganisms. Int Endod J. 2002;35(8):660-7.
16. Só MVR, Vier Pelisser FV, Darcie MS, Smaniotto DGR, Montagner F, Kuga MC. Pulp tissue dissolution when the use of sodium hypochlorite and EDTA alone or associated. Rev Odonto Ciênc. 2011;26(2):156-60.
17. Stojicic S, Zivkovic S, Qian W, Zhang H, Ha apa s a lo M. Ti s sue di s solut ion by sodium hypochlorite: effect of concentration, temperature, agitation, and surfactant. J Endod. 2010;36(9):1558-62.
18. Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(1):79-84.
19. Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemomechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J. 2006;39(6):484-92.
 Corresponding author:
Corresponding author:
Gustavo Golgo Kunert
Rua Florêncio Ygartua, 271 – sala 201 – Moinhos de Vento
CEP 90430-010 – Porto Alegre – RS – Brasil
E-mail: gustavogkunert@gmail.com
Received for publication: May 12, 2015
Accepted for publication: July 24, 2015













