Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.12 no.4 Joinville Out./Dez. 2015
ORIGINAL RESEARCH ARTICLE
Evaluating surfaces of titanium dental implants after contact with surgical gloves, steel rongeur, and titanium tweezers using scanning electron microscopy
Tatiana Miranda DeliberadorI; Caroline Moreira AuersvaldI; Maurício Santos de AraújoI; Luís Henrique ChavesI; Allan Fernando GiovaniniI; João César ZielakI
I Positivo University – Curitiba – PR – Brazil
ABSTRACT
Introduction and Objective: To use scanning electron microscopy (SEM) and determine whether the surfaces of titanium implants are damaged when touched with a steel rongeur, titanium tweezers, or surgical gloves. Material and methods: Ten dental implants were divided into five groups: Control (C), Titanium Tweezers (T-T), Steel Rongeurs (S-R), Surgical Gloves (S-G), and Steel Support (S-S). The implants were assembled in a metallic base (stub) with the aid of copper strips. They were then imaged and their microstructures were characterized using SEM. Results: An analysis of the obtained images showed that the implants that had been handled with titanium tweezers or a steel rongeur suffered some damage to their physical structure; "scratches" and other small signs of damage were visible on their surfaces. The affected areas were very small compared to the total surface area of the implants. Small dark local stains were observed at the spots where some of the implants had rubbed against a steel support. The rubbing of the implants against the support did not cause any structural damage. The implants handled with surgical gloves exhibited many dark stains their surfaces. This suggested that the powder from the surgical gloves had contaminated the surfaces of the implants. Conclusion: Using SEM imaging, it was determined that the surfaces of dental implants suffer minor physical damage when handled with various pieces of dental equipment. However, the damage should not result in failure of the osseointegration process. In vivo studies are needed to confirm this hypothesis.
Keywords: dental implants; osseointegration; scanning electron microscopy; transmission electron microscopy; titanium.
Introduction
The phenomenon of osseointegration, which was first described by Branemark in 1960 involves bone-implant interactions and is characterized by a direct, structural, and functional connection between the organized vital bone and the implant surface subjected to functional loads 7. The discovery of this phenomenon, which is also called functional ankyloses 15,17, has led to exciting and far-reaching developments in dentistry, since it has allowed for the replacement of lost dental elements 6.
Numerous studied have been performed on titanium dental implants, which are widely used clinically, with the aim of scientifically determining not only their compatibility with biological tissue but also whether they undergo osseointegration8 Titanium does not corrode and or cause allergic reactions 19.
Studies usually evaluate different formats, different surface treatments, different dental implant topographies and different alloys that constitute the implant 5,17. Of the various characteristics of dental implants, their surface topography is one of the most important one, as it determines the response of the adjacent tissue cells. This is because the surface topography has a significant effect on the migration, insertion, proliferation, and synthesis of collagen at the implant site and thus determines the type of tissue that will grow at the bone-implant interface as well as its degree of integration 12.
The surface treatment of dental implants results in an increase in the superficial roughness of the implants; this improves the odds of osseointegration and, hence, the success of the implants 4,12,14,18.
Various surface treatments have been described in the literature. In addition to those involving the incorporation of a hydroxyapatite surface layer and the use of a titanium plasma spray 9,13, the most common surface treatments are the acid etching of the machined surfaces of the implants, blasting the implants with particles with different hardnesses and sizes, and combinations of these two methods.
Osseointegrated dental implants are considered as a predictable modality of functional rehabilitation of partially and fully edentulous patients. The literature reports that more than 95% of dental implants have a lifespan of 10 years, while more than 92% of them have a lifespan of 15 years 1. However, in order to ensure that dental implants have long lifespans, meticulous planning and the use of the correct surgical technique are necessary 2. In addition, it is crucial to avoid touching the surface of the dental implant before it is inserted in the osseous bed, so that its surface is not contaminated and/or deformed. However, there are no studies on whether the surfaces of implants are really contaminated or deformed when touched. Thus, the aim of this study was to use scanning electron microscopy (SEM) to determine whether the surfaces of titanium dental implants are damaged when touched with a steel rongeur, titanium tweezers, or surgical gloves.
Material and methods
Ten dental implants (Attract, 4.3 x 13 mm, Lot No. 11093, Systhex) were used in this study. The implants were divided into five groups: Control (C), Titanium Tweezers (T-T), Steel Rongeur (S-R), Surgical Gloves (S-G), Steel Support (S-S). To analyze the microstructures of the implants using SEM, we assembled the implants in a metallic base (stub) with the aid of copper strips; no soldering was required. SEM system (JSM 6360-LV, JEOL, Japan) with magnifications varying from x50 to x3000 was used for the purpose. In the case of Group C, two implants were removed from their packaging with titanium tweezers without being touched on the surface and were immediately placed on a metallic base (stub) to be analyzed using the SEM system. The outer surfaces of the implants, which were the areas analyzed, were not touched at all. Further, it was ensured that no damage was caused to the implants. Thus, this group was used as a standard reference for comparison.
In the case of group T-T, two implants were removed from their packaging using titanium tweezers. The outer surfaces of both implants were pressed lightly, and the implants were immediately placed on a metallic base (stub) to be observed using SEM. In the case of group S-R, two implants were removed with a steel rongeur. Again, the outer surfaces of both were pressed lightly, and the implants were immediately placed on a metallic base (stub) to be observed using SEM.
In the case of group S-G, the two implants were handled using surgical gloves during the entire preparation process, that is, starting from their removal from the packaging until they had been glued to a metallic base in order to be observed using SEM. The outer surfaces of the implants were lightly pressed before the implants were placed on the base.
In the case of group S-S, two implants were removed from their packaging using titanium tweezers. A steel support is present around the implants inside the packaging in which the implants are stored. The implants were rubbed lightly against this support, in order to analyze the damage incurred by their outer surfaces.
Results
Group C
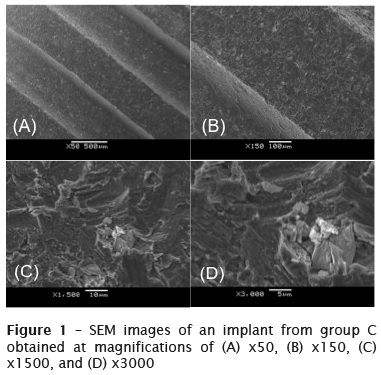
Figure 1 shows SEM images of the various implants obtained at magnifications of x50, x150, x1500, and x3000. Group C was used as the standard reference. All the changes that occurred in the physical structures of the implants in the other groups were analyzed with reference to the SEM images of the implants in group C. Figure 1 shows that a few white spots were present on the surfaces of the implants in group C. However, these spots did not imply a structural deficiency. On increasing the magnification, it was found that the spotted area was recessed and contained particles organized in a pattern.
Group T-T
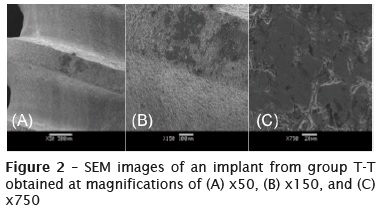
Figure 2 shows SEM images of an implant from group T-T obtained at magnifications of x50 to x750. A dark area was observed on the upper portion of the implant screw; this was probably caused by the pressure applied by the titanium tweezers on the area. By increasing the magnification, it was seen that the area was compact and exhibited a decrease in its superficial roughness as well as a small degree of irregularity.
Group S-R
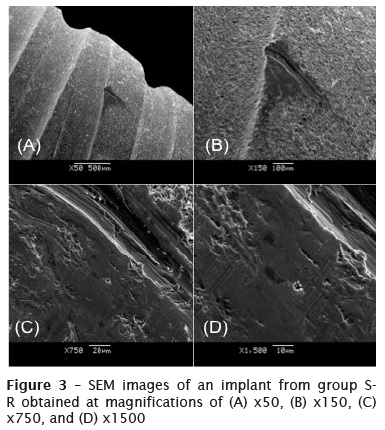
Figure 3 shows SEM images of an implant from group S-R obtained at magnifications of x50 to x1500. A groove can be seen on the surface of the dental implant; this was probably caused by the pressure applied by the steel rongeur on the surface. By increasing the magnification, it was seen that the binding structure of the particles surrounding the scratch had changed. The structure, which had earlier been rough, had become smooth, and the particles, which had earlier been separated from each other, were now arranged together now as if to form a single particle.
Group S-G
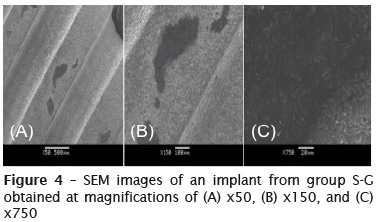
Figure 4 shows SEM images of a dental implant that had been handled with surgical gloves; the images have magnifications of x50 to x750 and show that the implant surface had a number of dark stains in different spots. These stains had irregular edges and were not deep. By increasing the magnification, it was observed that the surface of the implant was possibly contaminated, as the image was opaque, making it difficult to analyze the surface roughness and the structure of the particles in the stained areas.
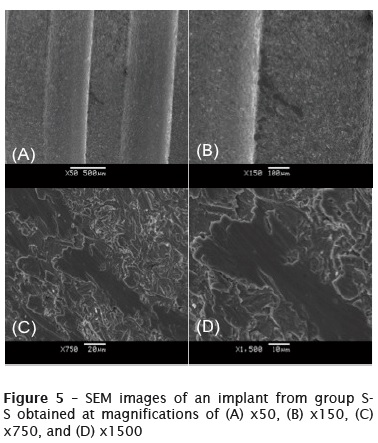
Group S-S
Figure 5 shows SEM images of an implant from group S-S obtained at magnifications of x50 to x1500. In these images, a black stain can be seen in a depressed region between the threads of the dental implant. This stain was probably caused by friction between the steel support and the implant. By increasing the magnification, it was seen that the particles in the stain had a changed structure. The rough surface of the implant had become smooth, while the particles in the stained area had aggregated, forming a single plate, and did not have any gaps between them.
Discussion
In this study, we used SEM to evaluate the surfaces of dental implants that had come in contact with different pieces of dental equipment. This was done as no such studies have been reported previously in the literature. Several studies have evaluated the effects of minor surface modifications on dental implants, with aim of optimizing the osseointegration process 8,10,11,13,14. It has been reported that such modifications accelerate the osseointegration process and improve its quality, resulting in greater bone deposition as well as a decrease in the period of repair 5,8,10-12,18,19. Therefore, any deformation of the implant surface can result adversely affect cell adhesion around the implants.
By analyzing the obtained SEM images of the implants from the various groups, it was found that the implants handled with a steel rongeur as well as those handled with titanium tweezers exhibited changes in their physical structure, including "scratches" and dark stains in isolated spots. This type of surface damage will probably not have a negative effect on the osseointegration of the implants, as the affected regions were very small when compared to the total surface area of the implants. It has been reported that once 60% of the implant area in contact with bone exhibits osseointegration, the success rate of the osseointegrated implant remains the same, with the implant exhibiting a lifespan of 1 to 18 years 3.
It was also noticed that the degree of deformation caused by the steel rongeur was greater than that caused by the titanium tweezers. This difference is attributable to the fact that steel is harder than titanium. However, it can be concluded that even titanium tweezers, which are recommended by manufacturers for handling dental implants, can cause a small degree of physical damage to the implant surface.
In the case of the implants rubbed against the steel support, small dark local stains were observed on the implant surface. Apparently, the rubbing of the implants on the support did not cause significant damage to the implant structure. Further, the small stains observed will probably not affect the outcome of the implant procedure adversely.
The implants in group C exhibited a homogeneous surface and satisfactory degrees of imbrication and roughness. Thus, these implants should osseointegrate perfectly in the implant area.
The most significant surface damage was observed in the case of the implants handled with surgical gloves. On analyzing the SEM images on these implants, it was found that the surfaces of the implants were covered with numerous dark stains. This suggested that the implant surfaces were probably contaminated with the powder that covers surgical gloves. This contamination can increase the failure risk of the osseointegration of the implants. However, in vivo should be performed to confirm this hypothesis.
Conclusion
Using SEM, it was determined that the surfaces of dental implants undergo minor physical damage when handled with various pieces of dental equipment.
Acknowledgments
The authors thank Systhex for donating the dental implants used in this pilot study.
References
1. Adell R, Eriksson B, Lekholm U, Brånemark PI , Jemt T. Long- term fol low-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347-59. [ Links ]
2. Albrektsson T, Wennerberg A. The impact of oral implants – past and future, 1966-2042. J Can Dent Assoc. 2005;71:327.
3. Albrektsson T, Zarb GA. Current interpretations of the osseointegrated response: clinical significance. Int J Prosthodont. 1993;6:95-105.
4. Baier RE, Meyer AE. Implant surface preparation. Int J Oral Maxillofac Implants. 1988;3:9-20.
5. Bowers KT, Keller JC, Randolph BA, Wick DG, Michaels CM. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int J Oral Maxillofac Implants. 1992;7:302- 10.
6. Brånemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3:81-100.
7. Brånemark PI, Zarb GA, Albrektsson T. Tissue integrated prostheses. In: Osseointegration in clinical dentistry. Chicago: Quintessence; 1985.
8. Carimo Marino LA, Deliberador TM, Zielak JC, Correr GM, Giovanini AF, Gonzaga CC. Microstructural and topographical characterization of different surface treatments of a surgical titanium alloy for dental implants. Implant Dent. 2012;21:207-12.
9. Coelho PG, Granjeiro JM, Romanos GE, Suzuki M, Silva NR, Cardaropoli G et al. Basic research methods and current trends of dental implant surfaces. J Biomed Mater Res B Appl Biomater. 2009;88:579-96.
10. Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2006;23:844-54.
11. Liu X, Chu PK, Ding C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng. 2004;27:49-12.
12. Nagem Filho H, Francisconi PAS, Laurito CJ, Fares NH. Influência da textura superficial dos implantes. Rev Odonto Ciência. 2007;22:82-6.
13. Novaes Jr. AB, Souza SL, de Oliveira PT, Souza AM. Histomorphometric analysis of the bone-implant contact obtained with 4 different implant surface treatments placed side by side in the dog mandible. Int J Oral Maxillofac Implants. 2002;17:377-83.
14. Piattelli A, Manzon L, Scarano A, Paolantonio M, Piattelli M. Histologic and histomorphometric analysis of the bone response to machined and sandblasted titanium implants: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1998;13:805-10.
15 . Schr o ede r A, Pohl e r O, Sut t e r F. Ti t an-Hohl z y l inde r impl ant a t mi t Ti t an- Spritzschichtoberfläche. Schweiz Mschr Zahnheilk. 1976;86:713-27.
16. Schroeder A, Stich H, Straumann F, Sutter F. Über die anlagerung von osteozement an eien belasteten implantatkörper. Schweiz Mschr Zahnheilk. 1978;88:1051-8.
17. Schwartz Z, Martin JY, Dean DD, Simpson J, Cochran DL, Boyan BD. Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. J Biomed Mater Res. 1996;30:145-55.
18. Shalabi MM, Gortemaker A, Van't Hof MA, Jansen JA, Creugers NH. Implant surface roughness and bone healing: a systematic review. J Dent Res. 2006;85:496-500.
19. Steinemann SG. Titanium – the material of choice? Periodontol 2000. 1998;17:7-21.
 Corresponding author:
Corresponding author:
Tatiana Miranda Deliberador
Universidade Positivo.
Rua Professor Pedro Viriato Parigot de Souza, 5.300 – Campo Comprido
CEP 81280-330 – Curitiba – Paraná – Brasil
E-mail: tdeliberador@gmail.com
Received for publication: July 12, 2015
Accepted for publication: September 30, 2015













