Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.13 no.2 Joinville Abr./Jun. 2016
ORIGINAL RESEARCH ARTICLE
Relationship of salivary flow of diabetic patients
Laís Santos PeresI; Dayani GalatoII; Gláucia Helena Faraco de MedeirosI
I Department of Dentistry, University of South Santa Catarina – Tubarão – SC – Brazil
II Department of Pharmacy, Brasília University – Brasília – DF – Brazil
ABSTRACT
Introduction: Diabetes mellitus is a highly prevalent health condition. Its systemic changes cause oral manifestations that may or may not be associated. Objective: To investigate the presence of xerostomia in patients with diabetes. Material and methods: This study consisted of a series of cases with 22 diabetic patients of the Family Health Strategy (FHS), neighborhood of Dehon, city of Tubarão (SC). A socio-demographic questionnaire was applied, the perception of dry mouth assessed, and the salivary flow measured at rest and after stimulation. To evaluate the association was adopted p<0.05. Results: 77% of patients had dry mouth feeling symptoms compatible with a diagnosis of xerostomia; of these, only 65% had stimulated salivary flow below the reference values (≤0.7ml/min) compatible with hyposalivation also demonstrating the association between xerostomia and hyposalivation. No statistically significant differences occurred between patients using xerostomic medication and decreased salivary flow when stimulated (p=0.15) and dry mouth sensation (p=0.30). Conclusion: The observed diabetic patients have a high prevalence of xerostomia, however, without association with any drug.
Keywords: diabetes mellitus; xerostomia; saliva.
Introduction
Diabetes mellitus (DM) is a chronic disease characterized by a disorder in the metabolism of insulin, causing hyperglycemia (decreased glucose entry into cells and increase in the blood), changes in the metabolism of fat, protein, and carbohydrates 25. DM is divided into type 1 and type 2. Type 1 DM occurs more in children and adolescents, the result of an autoimmune process in insulin-producing pancreatic cells. Type 2 DM is characterized by insulin resistance by their receptors or insufficient insulin secretion, most commonly expressed in obese adults; control is by oral hypoglycemic medications and diet 14. DM causes systemic and oral diseases, which may be signs or symptoms of the undiagnosed disease or of its aggravating symptoms 4.
The absence of metabolic control influences on the physiological changes that significantly reduce the immune capacity and inflammatory response, resulting often in decreased salivary peptide hormones as epidermal growth factor (EGF) present in saliva, a consequence of the decreasing or not in the salivary flow 21. When the patient does not present glycemic control, the susceptibility to oral manifestations of the disease increases. The dentist accounts to verify the presence of subsidies for early diagnosis, as well as control and monitoring of the disease both in regard to the DM as the oral diseases, often through a careful medical history and intraoral physical examination 12,14,25.
Although the patient is unaware of the disease or have no control of it, he/she is conducive to these oral alterations, the controlled patient may also present oral manifestations consistent with DM. Al-Maweri et al. 2 point out in their study that compensated diabetic patients had significantly higher prevalence of oral lesions than the control group. The oral lesions most commonly found in diabetic patients, controlled or not, are: periodontitis; gingivitis; fungal infections (candidose); healing difficulty; tooth decay; dry mouth and impairment of the salivary glands; ketone breath; change in shape, size, and texture the tongue; tooth loss; changed tooth development; altered taste; feeling of mucosal burning; and increased salivary glucose level 12,14,23.
Painful symptoms of burning sensat ion in the oral mucosa, halitosis, and decreased salivary viscosity and xerostomia (dry mouth) are characteristic symptoms of DM. Some studies report that xerostomia is present from 40% to 60% of uncontrolled diabetic patients. Carda et al. 7 reported the presence of 76%, of which 84.21% are uncontrolled diabetics. Xerostomia can be a result of decreased salivary flow, especially the parotid gland, or even in normal salivary flow conditions 4,9,17,18.
The sensation of dry mouth (xerostomia) and decreased salivary flow (hyposalivation) may be associated with or characterized to separately. DM causes polyuria (increased urine volume, to meet the need to eliminate excess sugar in the blood), which may affect the production of saliva (due to the excess of liquid excretion and consequently the salivary decrease and dry mouth). This condition greatly affects the diabetic patient's situation, as this calls for dry mouth while eating, difficulty in swallowing, requiring liquid aid, and mouth dryness overnight. Some complain of difficulty tasting, tongue pain and dryness while speaking, so being a problem not only physical but psychosocial 1,4,14.
Both xerostomia and hyposalivation can have their etiologies characterized by the use of xerostomic drugs used by diabetic and nondiabetic patients. Increasing life expectancy has led to increased use of medications that potentiate the xerostomic effect, especially those used for neurological purposes 4,8.
It is known that saliva plays an important role in maintaining oral health 15. In this context, the aim of this study was to verify the presence of xerostomia in diabetic patients, associated or not with hyposalivation.
Material and methods
This study was approved by the Research Ethics Committee of the University of Southern Santa Cata r ina under the CA AE number: 25192013.8.0000.5369, protocol number 668,700. All participants signed a consent form.
The study is characterized as a series of cases with 22 patients diagnosed with DM registered in the Unit of Family Health Strategy (ESF), neighborhood of Dehon, city of Tubarão (SC).
Inclusion criteria comprised: be over 18 years of age, absolute capacity, and DM diagnosis. Exclusion criteria were patients who did not have contact information in the medical record, syndromic, pregnant. and those who refused to participate in the study by not signing the Free and Clarified Consent Form.
Data collection was conducted through a questionnaire on sociodemographic questions, knowledge on oral and general health conditions adapted from Carda et al. 7. To assess the salivary flow, the methodology of Moura-Grece et al. 20 was applied: saliva at rest was collected in a plastic cup in which patients in first, swallowed the first saliva and expelled it for 5 continuous minutes; after so, stimulated saliva was obtained with latex pieces (1.0 cm thickness and 12 mm diameter) previously sterilized, patients expelled saliva into another plastic beaker for 5 minutes; after resting of the saliva to decrease foam interference, the saliva amount was quantified. Using a disposable 5 millimeter syringe, the volume was quantified and divided by 5 (collection time) to obtain the flow in ml/min. Values of ≥0.1 ml/min at rest and ≥0.7 ml/min for stimulated saliva were considered for hyposalivation 4,9,22,24. The values were recorded in their respective questionnaires.
Data were entered in an Excel™ spreadsheet and descriptive analysis by simple frequency was carried out. For statistical analysis, data were exported to the Statistical Package for Social Sciences (SPSS) version 20.0. The association tests were performed between the dry mouth variable (outcome) with exposure variables: use of medication, amount of saliva reported by the patient, and stimulated salivary flow. Chi-square test was adopted and, when relevant, Fisher exact test was used. The level of significance adopted was p<0.05.
Results
The socio-demographic profile of 22 patients with DM showed that 14 patients (63.6%) were female. The mean age of patients was 68.3 years, ranging from 49-88 years.
Of the 22 patients in the sample, eight (36.4%) have been diagnosed with DM for a year or less, and they were all the participants diagnosed with type 2 diabetes. Besides the diagnosis of diabetes, 18 patients (81.8%) also were diagnosed with high blood pressure (hypertension). The list of systemic diseases also diagnosed in these patients is shown in table I.
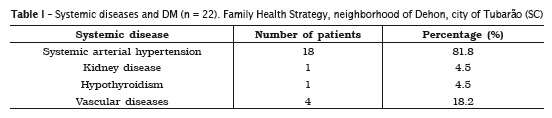
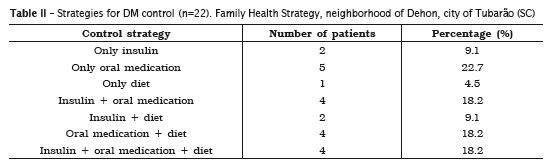
As regards the examination of glycated hemoglobin, in which the standard value standardized by the American Diabetes Association 2 is below 7.0% in diabetic patients, by adopting this criteria, 90.9% (20) of the patients were within the control value. Strategies for DM control reported by patients are shown in table II.
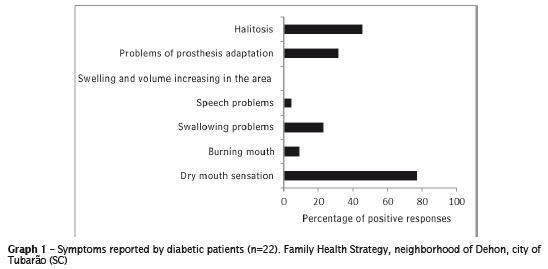
Of the respondents, eight (36.4%) reported to notice a reduced salivary flow, nine (40.9%) took medications that can cause some xerostomic effect and only eight (36.4%) had the collection of reduced salivary flow at rest (value ≤0.1 ml/min). However, when analyzing the stimulated salivary flow, 12 (54.5%) had salivary flow below the recommended value (≤0.7 ml/min). When asked about the symptoms, 17 patients (77.3%) reported feeling dry mouth. The ratio of symptomatology indicated by patients is seen the graph 1.
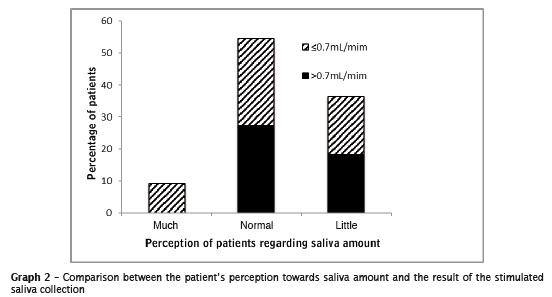
The relationship between salivary flow reported by patients (low, normal, and long) and collection of salivation is in graph 2.
The metabolic control analysis and dry perception of dry mouth together with a sample of stimulated salivary flow are shown in the graph 3.
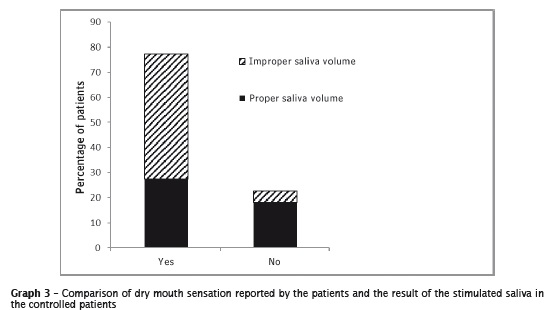
The associations between the variable dry mouth and amount of saliva (p=0.076) and with stimulated saliva (p=0.067) showed no significant difference. Similar, no significant difference was shown between the dry mouth sensation and the disease control (p=0.058) and the appropriate values of glycated hemoglobin (p=0.762).
The association of use of xerostomic drugs with the amount of saliva reported by patients did not show statistically significant results (p=0.546).
Statistically significant results were found in those that use xerostomic medication and had stimulated saliva in lower concentrations than the reference value (p=0.15).
The relationship between the use of xerostomic medication and the feeling of dry mouth reported by patients also showed statistically significant results (p=0.030).
Discussion
By its magnitude, the DM requires and develops an entire attention, care and monitoring cycle in their epidemiological profile, such as the risk factors of the disease, by the services responsible for health promotion in the country to have an effective control and prevent premature deaths from undiagnosed/uncontrolled disease.
The results obtained in this series of cases corroborate 9,16,23 those reporting that DM distribution occurs more in women and age 60 years or more, given that the majority of the sample consisted of women (64%) aged over 50 years. The higher incidence of DM diagnosis in women may be because, there is a resistance among men by seeking health, causing many not to seek appropriate care.
Considered the systemic disease that causes more morbidity in the world, insulin deficiency compromises some functions in the body that lead to the emergence of other systemic diseases, especially hypertension 16,23. The reported cases confirmed this statement, considering that all had at least one systemic disease together with diabetes, with prevalence of hypertension, and prior to or consequence of DM. Thus, besides the diagnosis and control of diabetes, also it is necessary to continue public policies to prevent the disease, because DM complications increase the morbidity and mortality numbers. Although a large number of people with diabetes do not have their diagnosis confirmed and still they do not have adequately control, the number of cases outlined here shows that diabetic patients treated at the institution have the diagnosis of the disease for several years to maintain, in general, DM controlled. This profile may be arising from policies in force in the country, particularly the Family Health, with periodic visits from community workers, which can control and check any changes earlier 5,6,10,16.
This study did not seek to assess oral health status of diabetic patients, although decompensated patients may have oral manifestations more than compensated individuals or those without DM 2,1,12,16,25. However, we sought to determine whether patients performed the control of insulin as recommended by SDM and ADA, especially glycated hemoglobin, considered the gold standard for check insulin control 3,5,9,16. Only one patient had this value above the recommended, although requested further examination as stated in the medical records. Because the test is done through laboratory blood sample, the request is made and registered in the medical records, and the patient should be tested and return to the doctor to register the new test result. This result demonstrates the commitment of both the patient and the professional team that is part of the unit.
The DM control affects the characteristic signs and symptoms of disease, as highlighted by Souza et al. 23. Although here the symptoms of dry mouth and glycated hemoglobin levels when related as a way to control the disease, did not reflect an association, one should be aware of the signs of salivary gland, as well as the condition of salivary flow for diabetic control maintenance 1. Even if the sample shows the relationship of dry mouth and how to control the disease (with diet or medication use – p=0.589) and the appropriate values of glycated hemoglobin (p=0.762) did not show association, one should be aware of the signs of salivary gland, as well as the condition of salivary flow for diabetic control maintenance.
It is known that old age can lead to decreased salivary flow, whether by physiological issues or the presence of any disease that, by itself, can cause damage to the salivary glands, or the need to use medicines that may reflect secondarily in such damage 1,8. In this study, we observed patients using xerostomic drugs and obtained decreased stimulated salivary flow and dry mouth. Even if the association between these variables did not show significant results, it is suggested a relationship with dysfunction of salivary secretion resulting from this situation. Of the sample, 54.5% exhibited decreased salivary flow, even that the association of dry mouth and saliva amount (p=0.076) and stimulated saliva (p=0,067) did not show statistically differences, corroborating the literature 4,7,11,17,19,23,26. Although further studies with larger samples should be conducted in the search for meaningful results, the relationship between the dentist and doctor is necessary, and the medical history of the patient is essential for diagnosis and best treatment in situations of hyposalivation and xerostomia.
The literature also reports that the symptomatology of xerostomia and/or hyposalivation can result in psycho-social problem for patients that have thus interfere strongly in the patient's routine 4,13,17. Concerning to symptomatology, all were marked, except for volume increase and edema of salivary glands, which reinforces the need for a dental-medical relationship
The results showed a prevalence of xerostomia in diabetic patients. However, it failed to find which factors specifically cause this condition. It is known that this condition may or may not be linked to hyposalivation, particularly in elderly patients. Thus, they suggest further studies with a larger number of diabetic patients to observe best associations, in order to obtain a statistically significant result and options for improving the quality of life of patients.
Conclusion
There is a high prevalence of xerostomia in diabetic patients treated at FHS Dehon, i.e., complaining of dry mouth. This occurs even when the disease is controlled. The sensation of dry mouth was confirmed in association with hyposalivation.
Even if the association between xerostomia and disease control was not observed, there was the presence of other predisposing factors such as the use of drugs that predispose such symptoms.
Even if there are study limitations by the sample size, this study showed that xerostomia is a common symptom in this population and that patients should be advised to seek dental care to investigate better the case and be attentive to the signs of salivation.
References
1. Aitken-Saavedra J, Rojas-Alcayaga G, Maturana- Ramírez A, Escobar-Álvarez A, Cortes-Coloma A, Reyes-Rojas M et al. Salivary gland dysfunction markers in type 2 diabetes mellitus patient. J Clin Exp Dent. 2015;7(4):e501-5. [ Links ]
2. Al-Maweri SA, Ismail NM, Ismail AR, Al-Ghashm A. Prevalence of oral mucosal lesion in patients with type 2 diabetes attending Hospital Universiti Sains Malaysia. Malays J Med Sci. 2013 Jul;20(4): 39-46.
3. American Diabetes Association. Tratament and cure/blood glucose control [cited 2014 Mar 3]. Available from: URL:http://www.diabetes.org/.
4. Andrades KMR, Oliveira GB, Ávila LFC, Odebrecht MR, Miguel LCM. Association of glycemic indexes, hyposalivation, and xerostomia type 1 diabetic patients. Int J Odontostomat. 2011;5(2):185-90.
5. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Diabetes mellitus. Brasília; 2006.
6. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Vigitel Brasil 2011: vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico. Brasília; 2012.
7. Carda C, Mosquera-Lloreda N, Salom L, Gomez de Ferraris ME, Peydró A. Structural and functional salivary disorders in type 2 diabetic patients. Med Oral Patol Oral Cir Bucal. 2006;11(3):9-14.
8. Chaves MJMC, Carneiro SDRM, Nobre ACL, Chaves MMC, Gomes FA, Lima DLF. Investigation of medicines with potential xerostomic effect used in institutionalized elderly. RSBO. 2015 Apr- Jun;12(2):191-5.
9. Cortelli JR, Pinheiro RMS, Costa FO, Aquino DR, Raslan AS, Cortelli SC. Salivary and microbiological parameters of chronic periodontitis subject with and without type 2 diabetes mellitus: a case- control study. Rev Odontol UNESP. 2014 May-Jun; 43 (3):196- 202.
10. Departamento de Atenção Básica. Saúde da família [cited 2014 May 5]. Available from: URL: http://dab.saude.gov.br/atencaobasica.php.
11. Donat FJS, Jordá LM, Mihi VM. Tratamiento de la boca seca: puesta al día. Med Oral. 2004; 9:273-9.
12. Eldarrat AH. Diabetic patients: their knowledge and perception of oral health. The Libyan Journal of Medicine. 2011;6:10.
13. Falcão DP, Mota LMH, Pires AL, Bezerra ACB. Sialometria: aspectos de interesse clínico. Rev Bras Reumatol, 2013 Nov-Dec;53(6):525-31.
14. Fernandes PM, Rocha CT, Peixoto ITA, Queiroz IF, Nelson Filho P, Queiroz AMD. Abordagem odontológica em pacientes com diabetes mellitus tipo 1. Pediatria. 2010 Oct-Dec; 32(4):274-280.
15. Franco G, Saab R, Pizzato LV, Torres MF, Fregoneze AP, Brancher JA. Analysis of salivar pH, flow rate, buffering capacity, concentrations of calcium, urea and total proteins in 2-8 years-old children with Down's syndrome. RSBO. 2014 Jan- Mar;11(1):66-70.
16. Freitas LRSD, Garcia LP. Evolução da prevalência do diabetes e deste associado à hipertensão arterial no Brasil: análise da Pesquisa Nacional por Amostra de Domicílios, 1998, 2003 e 2008 [cited 2015 Jul 11]. Epidemiol Serv Saúde. 2012;21(1):7-19. Available from: URL:http://scielo.iec.pa.gov.br/ scielo.php?script=sci_arttext&pid=S1679-49742 012000100002&Ing=pt&nrm=iso.
17. Harajanti K, Soebadi B, Mulyaningsi I. Prevalence of xerostomia on type 2 diabetes mellitus in Hajj Hospital Surabaya. Dent J. 2007;40(3):136-9.
18. Kakoi S, Hosseini B, Haghdoost A, Sanjari M, Gholamhosseinian A, Afshar VFN. Evaluation of salivary secretory immunoglobulin A levels in diabetic patients and association with oral and dental manifestatations. Sultan Qaboos University Med J. 2015 Nov;15(4):507-11.
19. Marinez RF, Jaimes-Aveldañez A, Hernández Pérez F, Arenas R, Miguel GF. Oral Candida spp carriers: its prevalence in patients with type 2 diabetes mellitus. An Bras Dermatol. 2013;88(2):222-5.
20. Moura-Grece PG, Assis VH, Cannabrava VP, Vieira VM, Siqueira TLD, Anaguizawa WH et al. Consequências sistêmicas da cirurgia bariátrica e suas repercussões na saúde bucal [cited 2015 Jul 11]. ABCD. 2012 Sep;25[3]:173-7. Available from: URL:http://www.scielo.br/scielo.php?script=sci_arttext&pid=S010267202012000300008&lng=en.
21. Paiva MDEB, Araujo AMM, Pluvezam MR, Costa HF, Costa LJ. Fluxo salivar e concentração do fator de crescimento epidérmico (EGF) na saliva de pacientes diabéticos tipo 2. Odontol Clín Cient. 2010 Jul-Sep;(3):235-7.
22. Soares MSM, Passos IA, Maia RMF, Costa LJ, Veloso DJ. Saúde bucal e sistêmica em idosos diabéticos. Rev Odontol Araçatuba. 2005;26[2]: 51-5.
23. Souza MGM, Costa ALL, Roncalli AG. Clinical study of the oral manifestations and related factores in type 2 diabetics patients. Brazilian J Otorhinolaryngol. 2011; 77(2):145-52.
24. Souza RR, Castro RD, Monteiro CH, Silva SD, Nunes AB. O paciente odontológico portador de diabetes mellitus. Pesq Bras Odontoped Clín Integr. 2003; 3[2]:71-7.
25. Visvanathan V, Nix P. Managing the patient presenting with xerostomia: a review. Int J Clin Pract. 2010;64[3]:404-7.
26. Yeh CK, Harris SE, Mohan S, Horn D, Fajardo R, Chun YH et al. Hyperglycemia and xerostomia are key determinants of tooth decay in type 1 diabetic mice. Laboratory Investigation. 2012;92:868-82.
 Corresponding author:
Corresponding author:
Laís Santos Peres
Avenida General Osório, n. 230
apto. 202 – Centro
CEP 95560-000
Torres – RS – Brasil
E-mail: lsperes01@gmail.com
Received for publication: December 8, 2015
Accepted for publication: April 29, 2016













