Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.13 no.2 Joinville Abr./Jun. 2016
LITERATURE REVIEW ARTICLE
Stem cells scaffolds as a carrier for tissue engineering
Thaysa Fedalto LopesI,II; Agnes LevandowskiII; Sabrina Cunha da FonsecaI; João Cesar ZielakI; Moira Pedroso LeãoI
I Department of Dentistry, Positivo University – Curitiba – PR – Brazil
II Department of Health and Biological Sciences, Positivo University – Curitiba – PR – Brazil
ABSTRACT
Introduction: Mesenchymal Stem Cells (MSCs) can be isolated from several body tissues, including dental tissues. As a result of being capable of differentiating into a variety of cell types, it can be presumed that stem cell therapy has an advantage when compared to other tissue repair methods. Objective: The aim of this paper is to provide a review about current and future materials for scaffolds to carry stem cells in tissue engineering in Dentistry, especially for bone tissue repair. Literature review: MSCs have great therapeutic potential in tissue engineering, they can be expanded in vitro, and combined with scaffolds they can be inserted into wounds to promote healing and tissue replacement. Conclusion: Stem cells from dental tissues have a real potential in Advanced Therapies. The combination of inductive scaffold materials with stem cells might optimize the approaches for bone regeneration. Although there are numerous available biomaterials potentially compatible to combine with MSCs, more studies need to be performed, due to the fact that for each indication there will be a more suitable material according to the wound's biological and mechanical requirement.
Keywords: stem cells; tissue engineering; tissue scaffolds.
Introduction
The potential therapeutic use of stem cells has been broadly researched in recent years. The ability to restore cells and tissue function without the need of immunosuppressive drugs and without the concern for tissue compatibility makes Mesenchymal Stem Cells (MSCs, usual acronym) a strong promise for the future. Until now, several progenitor cells derived from dental tissues have been isolated and characterized (table I).
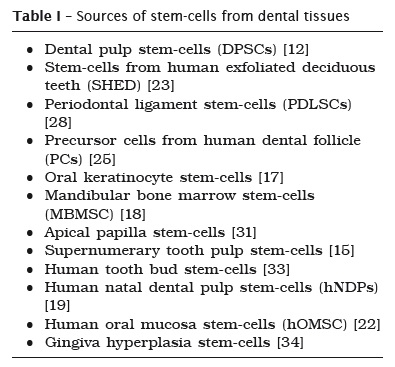
Stem cells derived from dental tissues are isolated from specialized tissues and have a strong ability to give rise to other cell lines, but with a different potential of bone marrow stem cells 16.
For stem cells to be used in tissue engineering a scaffold is essential to provide the necessary support for the transport of nutrients, oxygen and the elimination of metabolic waste 30, promoting a conducive environment for cell growth and differentiation.
The development of new biomaterials for tissue engineering provides a scientific basis for the creation of scaffolds that could provide appropriate regeneration and tissue repair 14.
The aim of this study is to provide a literature review of current and future materials for scaffolds to carry stem cells in tissue engineering, especially in the bone tissue regeneration.
Literature review
Stem cells are classified into two main: embryonic stem cells, which are found in the embryos and adult stem cells, found in adult tissues. Embryonic stem cells are present only in the early stages of development and are able to generate any cell type (Pluripotent Stem Cells). Adult stem cells are found in differentiated tissues and are able to generate specialized cells in some types of tissues (Multipotent Stem Cells). Adult stem cells can be isolated from many tissues in the human body, such as bone marrow, umbilical cord, periosteum, trabecular bone, adipose tissue, brain, dental pulp, pulp in deciduous teeth, the periodontal ligament, etc. 6.
MSCs have a clinical attractiveness because they are easy to expand, they have the ability to differentiate into various types of tissues 5, and can be directed to become osteocytes, chondrocytes or adipocytes. The high regeneration potential has aroused a great interest in the scientific community 3, due to its many clinical applications in cell therapy or tissue engineering.
Cell therapy is a therapy where cellular material is injected systemically or directly into the injured tissue, to promote local repair or to restore systemic health. Tissue engineering is the science that combines the principles of biology and engineering techniques in order to obtain biological substitutes for regenerating, replacing, modifying, repairing or restoring the function of organs and tissues.
The stem cells from exfoliated deciduous teeth (SHEDs) have a great potential for therapeutic use because of its differentiation capability and its easy access, since the collection is performed at the physiological exfoliation stage of the deciduous teeth. The tissues that used to be discarded may now serve as a basis for scientific research and clinical use in tissue regeneration and treatment of many diseases 23.
The easy access, the absence of ethical conflicts, and especially the potential for systemic use in tissue engineering and cell therapy motivate many researchers to better understand how these cells work and why they present peculiarities when compared in relation to stem cells found in other sources 16.
For tissue engineering use, it is essential that the cells are arranged in a matrix capable of facilitating the transport of nutrients, oxygen and the removal of metabolic waste 30. The matrix will promote a conducive environment for growth and cell differentiation.
Scaffolds can be classified as permanent or resorbable. Permanent scaffolds are stable in vivo, while the resorbable scaffolds are reabsorbed in vivo, metabolized by the body.
Cell scaffolds can be cultured in vitro to synthesize tissue and posterioly be implanted at the site of injury, where tissue or organ regeneration will be induced in vivo 26.
Scaffolds can be made of different types of materials, but there are a few considerations to create or to determine the suitability of a scaffold for tissue engineering. According to O'Brien (2011) 26, these considerations are biocompatibility, biodegradability, mechanical properties consistent with the anatomical site that will receive the scaffold, an architecture that enables cellular penetration and nutrients diffusion, and the technology used should enable a small scale production.
In large tissue defects, it is difficult to obtain repair and natural regeneration using only local cell administration, because in these situations not only the cells, but also the extracellular matrix and the surrounding environment may have been lost. Thus, to induce tissue regeneration, an artificial environment to the cells (scaffolds) may be used in order to assist in cell adhesion and consequent proliferation and differentiation 32.
Biomaterials play an important role in tissue regeneration, they will maintain the space for tissue growth and will also facilitate the integration with the host 8, substituting the extracellular matrix. Typically, three groups of biomaterials are used to manufacture scaffolds: ceramic, synthetic polymers, and natural polymers 26 (table II).
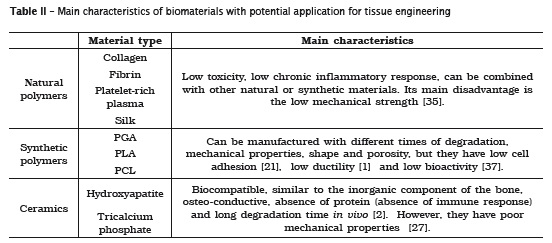
Ceramics scaffolds such as hydroxyapatite and tricalcium phosphate, have been widely used in bone regeneration because of their biocompatibility, similarity to the inorganic component of the bone, osteoconductiveness, absence of protein in their composition (providing absence of an immune response) and because of its long degradation time in vivo 2, which allows bone remodeling at the graft. Its disadvantages are low structural stiffness, inhibiling its use in regions of large mechanical stress, and its porous nature, which increases the risk of fractures 36. They are widely used in orthopedics and dentistry to repair bone defects, maintenance of the alveolar ridge and as well as orthopedic and dental implants 20.
Despite its advantages, the hydroxyapatite and tricalcium phosphate are not osteoinductive materials; hydroxyapatite is resorbed more slowly than the tricalcium phosphate, and their mechanical properties are poor, resulting in the inability to use on large bone defects which require stabilization 27. In such cases synthetic and natural polymers may be used to overcome these limitations. The most studied synthetic polymers for bone reconstruction include: polyglycolic acid (PGA), polylactic acid (PLA), and polycaprolactone (PCL) 2.
Natural biomaterials used in scaffolds may be made manufactured from components found in the extracellular matrix, such as collagen, fibrinogen, hyaluronic acid, etc., having an advantage of being biocompatible, and having mechanical properties similar to natural tissue 7.
The advantages of natural polymers include low toxicity and low chronic inflammatory response, in addition to the possibility of being combined with other natural or synthetic materials 35. These materials also provides a good environment for stem cell culture 7. Its disadvantages include low mechanical strength, difficult to handle and need for chemical modification 35.
Among the natural polymers, we can mention the platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). The platelet concentrates are widely used in tissue regeneration because of its high content of growth factors and their easy processing 9. The combined use of stem cells and platelet concentrates causes an enrichment of the culture medium by the growth factors secreted, enhancing the capacity of tissue regeneration 24, but it has low stability for regenerative medicine 29.
Each tissue needs specific requirements, that will depend on the type of cell, the site, its function, and its mechanical properties. For bone remodeling, it is necessary that the scaffold material has a porosity and can be produced in a threedimensional anatomic shape 35. Best results were obtained with the combination of MSCs and porous ceramics, in where the properties of ceramics were integrated with the properties of osteoprogenitor cells to form a vital and vascularized bone tissue, with biomechanical properties 4.
The success of osteoinductive grafts depends on the efficiency of the scaffold material. The ideal scaffold should increase the exposure of the host tissue to the growth substance and ensure uniform distribution. It should be safe, biocompatible, biodegradable, and resorptive as bone formation occurs 13.
Discussion
Stem cells are undifferentiated cells capable of unlimited self-renewal and able to differentiate into several cell types. Stem cells have a great therapeutic potential and are also a promise to regenerate and restore a tissue's normal function 10.
The potential capacity for MSCs to differentiate, the possibility of grafting, its immunosuppressive effects and the possibility of culture expansion, led to a clinical interest in its use via intravenous infusion or local administration in different pathological situations 4.
The scaffold design is critical, since it must be capable of supporting cell adhesion and proliferation, and also have favorable mechanical properties. In addition, there are parameters that must be considered when manufacturing a scaffold, such as porosity, mechanical integrity and the effect of surface morphology in cell adhesion and proliferation 38.
Currently, the autogenous bone graft has been used as the gold standard for bone repair and replacement. However, the use of this type of graft has several disadvantages, including cost, trauma, the possibility of donor site morbidity, limited availability and resorption. These disadvantages can be eliminated by the use of allografts, which are obtained from living donors or tissue banks. Despite increased availability and donor site morbidity disposal, this type of graft is associated with the transmission of diseases, bacterial infection, and immune rejection, in addition to presenting a limited osteoconductiveness 11. Thus, the creation of the ideal material for bone replacement is necessary 20.
The selection of the biomaterial to be used in a scaffold is very important; in addition to being biocompatible, promote chemical stability, having good physical properties, controlled degradation, allowing cell adhesion and proliferation, the biomaterial should mimic completely or partially the extracellular matrix of the tissue that will be replaced 38.
One of the major advantages of t issue engineering is the possibility of creating a tissue that corresponds exactly to the hosts' requirements in size, shape, and immunological compatibility, minimizing the need for further treatment 35.
Conclusion
Stem cells point to a promising future in dentistry to replicate dental tissues, in order to repair or recover them, especially in endodontics, periodontics and surgery (grafting).
The use of biomaterials in bone t issue engineering has many advantages over traditional surgical procedures. The possibility of using biomaterials to repair large bone defects may replace the need for traditional grafting surgery, providing a new method for bone repair. Among all the biomaterials for scaffolds, ceramics are the most appropriate when bone tissue regeneration is needed, especially hydroxyapatite and tricalcium phosphate.
Although studies with stem cells are quite recent, they have a great potential. It is expected that in the future the isolation of stem cells from different body parts will be possible, and, along with appropriate scaffolds, MSCs could be uses in the treatment of numerous disorders in dentistry and medicine.
References
1. Abdal-Hay A, Hussein KH, Casettari L, Khalil KA, Hamdy AS. Fabrication of novel high performance ductile poly(lactic acid) nanofiber scaffold coated with poly(vinyl alcohol) for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2016;60:143-50. [ Links ]
2. Abukawa H, Papadaki M, Abulikemu M, Leaf J, Vacanti JP, Kaban LB et al. The engineering of craniofacial tissues in the laboratory: a review of biomaterials for scaffolds and implant coatings. Dent Clin North Am. 2006;50(2):205-16, viii.
3. Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23(20):4131-41.
4. Cancedda R, Dozin B, Giannoni P, Quarto R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003;22(1):81-91.
5. Chagastelles PC, Nardi NB. Biology of stem cells: an overview. Kidney Int Suppl. 2011;1(3):63-7.
6. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all postnatal organs and tissues. J Cell Sci. 2006.119(Pt 11):2204-13.
7. Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60(2):215-28.
8. De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4-5):292-307.
9. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-67.
10. Fodor WL. Tissue engineering and cell based therapies, from the bench to the clinic: the potential to replace, repair and regenerate. Reprod Biol Endocrinol. 2003;1:102.
11. Garcia-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112-21.
12. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25):13.625-30.
13. Gurevich O, Vexler A, Marx G, Prigozhina T, Levdansky L, Slavin S et al. Fibrin microbeads for isolating and growing bone marrow-derived progenitor cells capable of forming bone tissue. Tissue Eng. 2002;8(4):661-72.
14. Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295(5557):1.014-7.
15. Huang AH, Chen YK, Lin LM, Shieh TY, Chan AW. Isolation and characterization of dental pulp stem cells from a supernumerary tooth. J Oral Pathol Med. 2008;37(9):571-4.
16. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792-806.
17. Izumi K, Tobita T, Feinberg SE. Isolation of human oral keratinocyte progenitor/stem cells. J Dent Res. 2007;86(4):341-6.
18. Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13(4):767-73.
19. Karaoz E, Dogan BN, Aksoy A, Gacar G, Akyuz S, Ayhan S et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2010;133(1): 95-112.
20. LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;(395):81-98.
21. Li X, Ding J, Wang J, Zhuang X, Chen X. Biomimetic biphasic scaffolds for osteochondral defect repair. Regen Biomater. 2015;2(3):221-8.
22. Marynka-Kalmani K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA et al. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells. 2010;28(5):984-95.
23. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100(10):5.807-12.
24.Morschbacher PD, Alves Garcez TN, Paz AH, Magrisso AB, Mello HF, Rolim VM et al. Treatment of dilated cardiomyopathy in rabbits with mesenchymal stem cell transplantation and platelet-rich plasma. Vet J. 2016;209:180-5.
25.Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24(2):155-65.
26. O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Materials Today. 2011;14(3):88-95.
27. Oreffo ROC, Triffitt JT. Future potentials for using osteogenic stem cells and biomaterials in orthopedics. Bone. 1999;25(2, Supplement 1): 5S-9S.
28. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429): 149-55.
29. Shimojo AAM, Perez AGM, Galdames SEM, Brissac ICdS, Santana MHA. Performance of PRP associated with porous chitosan as a composite scaffold for regenerative Medicine. The Scientific World Journal. 2015;2015:396131.
30. Soares AP, Knop LAH, Jesus AA, Araújo TM. Células-tronco em odontologia. Revista Dental Press de Ortodontia e Ortopedia Facial. 2007;12:33-40.
31. Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34(2):166-71.
32. Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface. 2009;6 Suppl 3:S311-24.
33. Takeda T, Tezuka Y, Horiuchi M, Hosono K, Iida K, Hatakeyama D et al. Characterization of dental pulp stem cells of human tooth germs. J Dent Res. 2008;87(7):676-81.
34. Tang L, Li N, Xie H, Jin Y. Characterization of mesenchymal stem cells from human normal and hyperplast ic gingiva. J Cel l Physiol . 2011;226(3):832-42.
35. Vats A, Tolley NS, Polak JM, Gough JE. Scaffolds and biomaterials for tissue engineering: a review of clinical applications. Clin Otolaryngol Allied Sci. 2003;28(3):165-72.
36. Wan DC, Nacamul i RP, Longaker MT. Craniofacial bone tissue engineering. Dent Clin North Am. 2006;50(2):175-90, vii.
37. Yunus Basha R, Sampath Kumar TS, Doble M. Design of biocomposite materials for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2015;57:452-63.
38. Zhang L, Morsi Y, Wang Y, Li Y, Ramakrishna S. Review scaffold design and stem cells for tooth regeneration. Japanese Dental Science Review. 2013;49(1):14-26.
 Corresponding author:
Corresponding author:
Moira Pedroso Leão
Rua Prof. Pedro Viriato Parigot de Souza, 5.300
Bloco Marrom – sala 111 – Campo Comprido
CEP 81280-330
Curitiba – PR – Brasil
E-mail: moirapedroso@gmail.com
Received for publication: February 12, 2016
Accepted for publication: March 23, 2016













