Services on Demand
Article
Related links
Share
RSBO (Online)
On-line version ISSN 1984-5685
RSBO (Online) vol.13 n.4 Joinville Oct./Dec. 2016
LITERATURE REVIEW ARTICLE
AH Plus extrusion into periapical tissue: literature review of main related properties and report of clinical cases
Karen Lys HubbeI; Kauhanna Vianna de OliveiraI; Beatriz Serrato CoelhoI; Flares Baratto-FilhoI
I Department of Dentistry, Positivo University – Curitiba – PR – Brazil
ABSTRACT
Introduction: The sealers were developed for filling of root canals. Due to their physicochemical and technical properties used for obturation, often, extrusion is observed through apical constriction and occasionally by lateral and secondary canals. Objective: To review the literature on important properties to be considered in AH Plus sealer extrusion and report a case series of this sealer extrusion. Literature review: Articles evaluating the cytotoxicity and biocompatibility properties, besides flow and solubility were selected. Case report: In the presented cases, endodontic treatment was performed with rotary instrumentation and irrigation with 2.5% NaOCl. Obturation employed visual, tactile, and radiographic proof of gutta-percha main cone, and different obturation techniques. There were no reports of pain during and after endodontic treatment. Conclusion: AH Plus has adequate properties for a filling material and causes no major damage to the periapical tissues due to its little cytotoxic.
Keywords: Endodontics; physical and chemical properties; root canal obturation.
Introduction
In endodontic therapy, obturation aims to avoid the recontamination of the already cleaned canal by filling with gutta-percha points and endodontic sealer 4,7,16-18,25. The endodontic sealers have many functions during the obturation of the root canal system: lubrication and aid in point seating, union among the gutta-percha points and the root canal walls, and filling of empty spaces not completed by the main material. For the best fulfilling of the functions of the sealer, some physicochemical and biological properties are desirable: adherence to canal walls even in presence of moist; dimensional stability; biocompatibility with the periapical tissues; good working time; good flow to fill narrow canals, inaccessible to biomechanical preparation; easy application inside the canal; bactericide; bacteriostatic 22.
A wide range of sealers with di f ferent chemical compositions, properties, and practical characteristics is available in dental market 21. Today, we found oxide zinc/eugenol, calcium hydroxide, resin, and glass ionomer based sealers 4. Among them, plastic resin based sealers is increasingly being used in Dentistry 17.
Developed by Dentsply (1997), AH Plus was launched into the market as two pastes, packaged in tubes (of 4 ml each), and composed by epoxy resin and amines. According to the manufacturer, AH Plus offers longer sealing duration, great dimensional stability, high radiopacity, polymerization without forming formaldehyde, and self-adhesive properties. AH Plus has a working time of 4 hours at 23°C, and setting time of 8 hours at 37°C.
The endodontic sealers are developed to be inside the root canal system, but frequently extrusion out of the root canal system is observed through the apical constriction and occasionally through lateral and secondary root canals. Thus, it is imprescindible that the sealer used is as most biocompatible and as least cytotoxic as possible 26.
This study aimed to review the literature on cytotoxic/biocompatibility, flow, and solubility of AH Plus sealer, important properties to be considered in the extrusion of sealer as well as to report a case series of AH Plus extrusion with clinical and radiographic following-up.
Literature review
Cytotoxic/biocompatibility
Biocompatibility is the ability of the material to be compatible with the live tissues. It is one of the factors that influences the professional to choose the sealer to be in contact with the periapical tissues. The biocompatibility of the materials is generally evaluated by ex vivo cytotoxic and in vivo implant techniques 25.
Batista et al.. 3 developed a study to evaluate histologically the inflammatory reaction of Endofill, Endomethasone, Sealer 26, and AH Plus sealers, through subcutaneous implants in the conjunctive tissue of rats. The inf lammatory reaction was verified after 7, 14, and 30 days of implant permanence. In the study, all sealers developed an inf lammatory reaction in the conjunctive tissue, whose intensity varied according to the type of sealer used, material amount, and time of implant permanence. Among the four sealers, Endomethasone exhibited the best biological behavior, followed by Sealer 26 and AH Plus.
Sari and Duruturk 20 developed a 4-year study evaluating the clinically and radiographically the effects of non-intentional extrusion of AH Plus sealer in the healing of permanent teeth with apical periodontitis. The following-up was performed 3 and 6 months, and then 4 years after the endodontic treatment. Of 49 canals with extrusion of AH Plus sealer, 41 showed complete healing.
Kangarloo et al.. 11 tested the cytotoxic of four sealers (AH Plus, Sankin, Tubliseal EWT, and Apexit), in fibroblast of rats in culture medium. The four sealers were in contact with the cellular culture at two moments: just after the handling of the sealers and three hours after the setting. AH Plus in contact with the fibroblasts three hours after handling showed initial cytotoxic increase after the first 24 hours that considerably decreased after 7 days, reaching the level of the control group; this sealer also had the smallest results of stimulation of interleukin production and consequently the smallest inflammatory reaction against the substances of AH Plus formula. AH Plus and Sankin sealers showed low cytotoxic, while Tubliseal EWT sealer had the highest levels of inflammation and cell toxicity.
Tanomaru-Filho et al.. 23 conducted a study to evaluate the process of periapical healing after root canal filling with Intrafill, AH Plus, Roeko Seal, and Resilon/Epiphany system sealers. In 64 dog roots, the sealer extrusion was induced. Elapsed 90 days, the animals were killed and the samples histologically analyzed to evaluate the intensity and extension of the inf lammatory infiltrate, thickness of the periodontal ligament, bone, and apical cementum, resorption, and sealing of the apical opening. Intrafill sealer showed the least favorable histopathological results in apical repair (with severe inflammatory reaction and periodontal ligament thickening) than the other sealers (AH Plus, Roeko Seal, and Resilon/Epiphany), which exhibited similar and satisfactory results.
Hiyasat et al.. 10 tested the cytotoxic of four resin-based sealers (AH Plus, EndoREZ, Epiphany, and Metaseal), in fibroblast culture. The sealer cytotoxic was smaller in AH Plus; followed by EndoRez, with moderate cytotoxic; Epiphany, with high cytotoxic; and Metaseal, severely toxic.
Portela et al.. 17 tested the biocompatibility of three segments of AH Plus sealer (initial, medium, and final portion) through subcutaneous implants on the back of the rats. The histologically evaluation was performed after 30 and 90 days of implant. The authors did not observe significant differences in the inflammatory reactions of the many portions and in the total homogenization of the AH Plus sealer. Also, they did not classify the tested sealer as biologically compatible within the established parameters and experimental conditions.
Because of controversial findings in a literature review, Willershausen et al.. 26, compare in vitro the biocompatibility of four sealers (GuttaFlow, Endosequence BC, Pulp Canal Sealer EWT, and AH Plus Jet). The tests were performed in fibroblasts from human periodontal ligament. AH Plus Jet sealer in contact with culture medium showed a significantly higher cytotoxicity than the other tested sealers and control group.
Scelza et al.. 21 tested the biocompatibility of the sealers Real Seal SE, AH Plus, GuttaFlow, Sealapex, Roth 801, and ThermaSeal Plus on human gingival fibroblasts. AH Plus showed the cytotoxicity effect after the first day that decreased after 7, 14, 21, and 28 days.
Ehsani et al.. 8 tested the cytotoxicity of two amine epoxy resin based sealers (AH Plus and 2Seal) on osteoblast-lie cells. Both sealers demonstrated significant cytotoxicity. The cytotoxicity of AH Plus increased over time from 24 to 72 hours of evaluation.
An in vitro study executed by Parirokh et al.. 16 in which human gingival fibroblasts were submitted to the direct contact with fluoride varnish (Duraflur) and two endodontic sealers (AH 26 and AH Plus) at two different dilutions. The study aimed to compare the cytotoxicity of the varnish and the sealers. The cell viability of the gingival fibroblasts was significantly higher on the AH Plus than on AH 26 sealer and Duraflur varnish.
Konjhodzic-Prcic et al.. 13 tested the cytotoxicity of four sealers (GuttaFlow, AH Plus, EndoRez, and Apexit) in direct contact with human gingival fibroblast on culture medium. The cell viability after the contact with freshly-mixed AH Plus was of 94.3% of live cells; after 24 hours of 95.1%; after 48 hours of 96.4%; and after 7 days of 75.9%. These results showed that AH Plus cytotoxicity is a little higher than that after 7 days; however, the decreasing in the number of live cells was within the limits of insignificance in the cytotoxicity scale.
Candeiro et al.. 6 tested the cytotoxicity and genotoxicity of AH Plus and Endosequence BC sealers in culture medium of human gingival fibroblasts. The cell viability was measured at 1, 3, 5, and 7 days. The cytotoxicity (cell viability) and genotoxicity (alteration in the genetic material of the organism) was higher in Endosequence BC than in AH Plus sealer.
In the study of Wei and Bing 25, samples containing iRoot SP, Pro Root MTA, and AH Plus sealers were placed into the subcutaneous tissue of the back and tibia bone of 36 rats, which was killed for histological evaluation after 7, 30, and 60 days. The study aimed to observe the inflammatory reaction of the conjunctive tissue and bone formation in contact with the selected sealers. AH Plus sealer showed the largest infiltrate of inflammatory cells in contact with the subcutaneous conjunctive tissue compared with the bone tissue.
Flow
The endodontic sealer flow characterizes the capacity of passing through the gutta-percha points and the dentinal wall, filling the empty spaces and ramifications, inaccessible to the solid material.
Alonso et al.. 2 tested the f low of two endodontic sealers (Endofill and AH Plus). In the study, AH Plus showed the highest flow capacity than that of Endofill.
Sydney et al.. 22 verified the vertical flow of N-Rickert, Endofill, Zinc Oxid and Eugenol, AH Plus, EndoRez, and Intra-Fill sealers. It was used 0.1 ml of each sealer placed with the aid of a syringe on the top of a millimetric vertical glass plate. The flow was assessed at different times. Of the tested sealers, Endofill exhibited the highest flow, followed by N-Rickert and AH Plus. Intrafill, Zinc Oxid and Eugenol, and EndoRez did not show flow over the study.
Some factors may influence on the flow capacity of the sealer, among them: the temperature, humidity, and powder/liquid proportion. To test these factors of the AH Plus, AH 26, and Endofill sealer, Khedmat et al.. 12 conducted a study through oscillatory and dynamic shear bond strength to verify the effect of temperature changes on the viscoelastic behavior of the sealers. The experiments were conducted at 25°C (environmental temperature) and 37°C (mouth temperature). At both temperatures, AH Plus behaved as solid viscoelastic, that is, the flow capacity and elasticity did not change from the handling at 25°C to the placement into root canal at 37°C. The same occurred with Endofill. AH 26 sealer was more sensitive to temperature changes, behaving as liquid at environment temperature and changing to solid at body temperature.
Viapiana et al.. 24 investigated the effectiveness of the filling of root canals and tridimensional sealing of BioRoot RCS sealer in comparison with AH Plus sealer. AH Plus exhibited a higher percentage of root canal filling than that of BioRoot RCS sealer. AH Plus sealer completely penetrated the dentinal sealers at the cervical and medium thirds, but in small amount at the apical third.
Ormiga et al.. 15 compared the flow capacity of AH Plus, Pulp Canal Sealer, and EndoRez sealers on the ramifications of the three thirds of premolar roots. All sealers demonstrated adequate flow capacity and could fill the ramifications. However, EndoRez sealer significantly filled a greater number of ramifications than that of AH Plus and Pulp Canal Sealers.
Solubility
The solubility is the capacity of the substance must dissolve into another. Marin-Bauza et al.. 14 tested the setting time, f low, radiopacity, solubility, and dimensional alteration of six endodontic sealers (AH Plus, Polifil, Apexit Plus, Sealapex, Endomethasone, and Endofill). AH Plus sealer showed characteristics within the guidelines established by ANSI/ADA.
Cañadas et al.. 5evaluated the physicochemical properties, radiopacity, pH, and solubility of Endo CPM Sealer, Activ GP, and Sealapex, compared with those of AH Plus sealers. Concerning to the solubility, all materials meeting the requirements of ANSI/ADA, that is, solubility lower than 3% of the weight, without statistically significant differences among the sealers.
Borges et al.. 4 compared two sealers: MTA FillApex and AH Plus by testing the properties of solubility, pH, and radiopacity. AH Plus showed the smallest value of solubility.
Clinical case reports
Clinical case #1
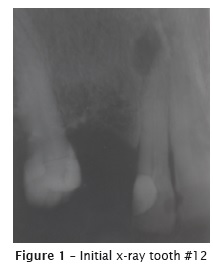
Patient L.C., female, aged 70 years were referred to endodontic retreatment of the right maxillary lateral incisor (#12). for prosthetic reasons. At clinical examination, the tooth was asymptomatic, without fistula, but with crown darkening. Radiographically, root canal had unsatisfactory filling material and chronic apical lesion (figure 1).
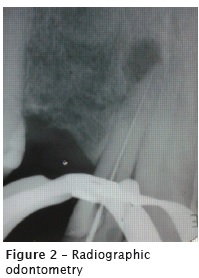
At the first appointment, the endodontic opening was achieved with burs no. 1012 and 3080. The filling material was removed from the cervical and medium thirds with Gates Glidden burs no. 1, 2, and 3, together with K files (Maillefer-Dentsply, Ballaigues, Switzerland). Following, electronic odontometry was performed with Romiapex A-15 device (Romidan, RJ, Brazil), and proved with the radiograph (figure 2). The root canal emptying was completed up to apical foramen with K files. The root canal was prepared with crown-apex technique, obtaining the surgical diameter of size #40 (K file). During all root canal preparation, root canal was irrigated with 2.5% sodium hypochlorite (NaOCl). Intracanal medication was Propylene glycol for 18 days.
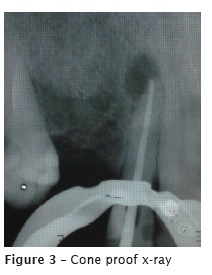
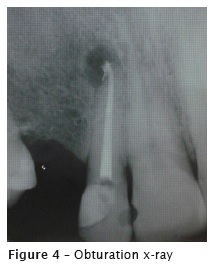
At the second appointment, the intracanal medication was removed with the aid of hand files and NaOCl. The canal was reprepared with the aid of Wave One Large size #40.08 (Maillefer- Dentsply, Ballaigues, Switzerland). Final irrigation was performed with 17% EDTA for 5 minutes, agitated with the aid of Easy Clean (Easy, Belo Horizonte, Brazil), at reciprocating mode, for three cycles of 20 seconds each. Following, the root was irrigated with 2.5% NaOCl and dried with absorbent paper points. At the same appointment, the main cone was proved (figure 3) and the tooth was filled through thermoplasticization with single cone Wave One Large size #40.08 (Dentsply, Ballaigues, Switzerland) and AH Plus sealer (Dentsply, Ballaigues, Switzerland). On the final radiograph (figure 4), the extrusion of the endodontic sealer was observed. The patient was asymptomatic at filling procedure and posttreatment following-up.
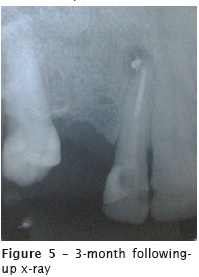
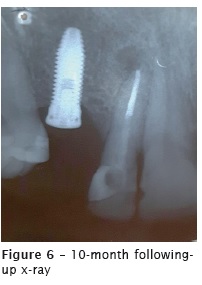
The first following-up appointment was performed 88 days after the root canal filling. The tooth already had a glass fiber core and post cemented by the prosthesist. Radiographically, the apical lesion radiolucency and size reduced, but the radiopacity of the sealer extrusion did not disappear (figure 5). The tooth remained asymptomatic during all this period. Eight days after the first followingup x-ray, the patient was submitted to implant installation at the area of the right maxillary canine. The implantodontist performed the curettage of the lesion and extruded material, without other intervention on tooth #12 (figure 6).
Clinical case #2
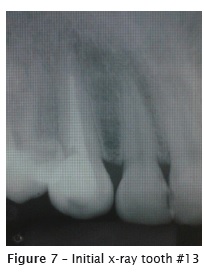
Patient A. L., female, aged 34 years were referred by the prosthesist to endodontic retreatment of right maxillary canine (#13). Clinically, the crown was darkened and asymptomatic. Radiographically, root canal filling was unsatisfactory.
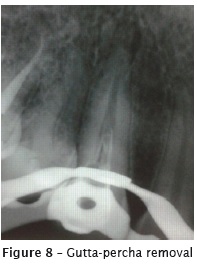
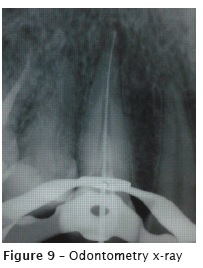
At the first appointment (figure 7), after crown opening, the gutta-percha was removed with D-Race rotary files (FKG, Switzerland): DR1 #30.10 (1000 RPM/1.5N), used at the cervical third of the root canal (straight portion); and DR2 #25.04 (600 RPM/01N), used at the medium and apical thirds, without solvent, with pecking movements. Two xrays were taken to verify the presence of remaining gutta-percha (figure 8). Then, the remaining material was removed with K files and solvent (Eucalyptol, Biodinâmica, Ibiporã – PR, Brazil). The radiographic odontometry (figure 9) was complemented with the electronic odontometry (Romiapex A-15 device). The intracanal medication was tricresol.
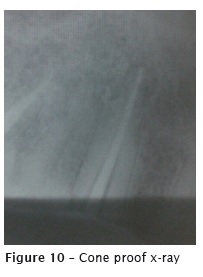
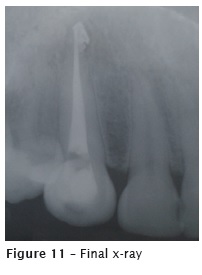
The second appointment occurred one week later. Due to the canal amplitude, the crown-apex preparation was performed with K files up to size #40 and copiously irrigation with 2.5% sodium hypochlorite and 17%EDTA. After the root canal drying, the main cone was proved (figure 10), followed by the obturation with single cone Wave One Large #40.08, accessory points, and AH Plus sealer, through lateral condensation technique (figure 11).
AH Plus sealer extrusion towards apical area occurred after the vertical condensation of guttapercha with Paiva's condenser. At that moment, the patient reported no discomfort during or after obturation.
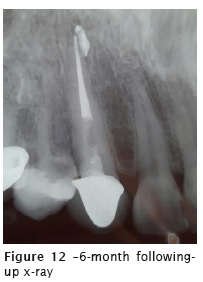
Six months after the endodontic treatment, a following-up x-ray was taken (figure 12). On the image, we noted the movement of the sealer inside the chronic lesion, but without reduction of the radiopacity. The tooth was asymptomatic.
Clinical case #3
Patient L.S., male, aged 34 years, was referred to endodontic treatment after daily routine appointment due to the presence of active fistula on the area of the left maxillary lateral incisor (#22). Clinically, the crown had a mild darkening with a mesialdistal- palatal resin restoration. The patient did not complain about pain. Radiographically, the image showed a chronic lesion on the root apex.
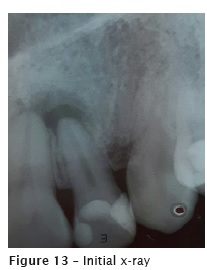
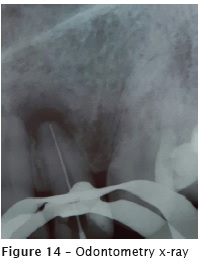
After the crown opening, the canal emptying was performed with files at decreasing order, starting with K file size #40 (figure 13). Next, the electronic odontometry was executed (Romiapex A-15 device) and confirmed with the radiograph (figure 14). The crown-apex preparation was concluded with reciprocating file Wave One Large #40.08. Intracanal medication was a paste of calcium hydroxide with Propylene glycol for 50 days. During all biomechanical preparation, the tooth was irrigated with 2.5% NaOCl and 17% EDTA.
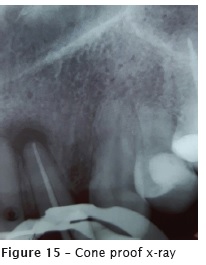
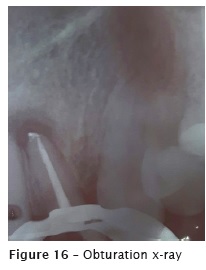
After the fistula remission and no symptomatology, the endodontic filling was accomplished with thermoplasticization through single cone Wave One Large #40.08 (figure 15) and AH Plus sealer (figure 16).
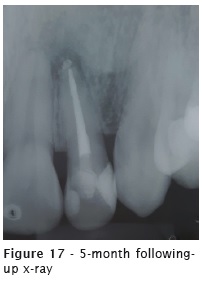
The patient reported no pain or discomfort after the radiographic image of the extrusion. Five months after the obturation, a following-up x-ray was taken (figure 17), revealing the reduction of the radiolucency of the apical lesion. The tooth was asymptomatic.
Discussion
The resin-based sealer appears as innovation in Endodontics. They were developed to achieve the best clinical results in the filling of root canal systems. AH Plus is one of the most modern resinbased sealers 2.
The accidental extrusion of the endodontic sealer towards the periapex is a common event. The direct contact of the sealer with the periapical tissues for longer periods can cause chronic inflammatory reaction 6,20,26. The studies of Wei and Bing25 and Canedo et al.. 7 observed that after 6 months, macrophages phagocyte the sealer particles leaving them to the peripheral areal of the inflammation, eventually completely cleaning the extruded sealer.
The intensity of the inflammatory response can also be determined by the amount of extruded sealer. Although many of these studies did not rigorously reflect the inflammatory reaction in the apical tissues, they are valid because aid in evaluating the irritation potential of the endodontic sealers 3.
Cytotoxicity is an important property that should be considered during the sealer choice due to the contact with the periodontal ligament cells. The toxicity of a sealer may damage the periapical tissue and even provoke alterations in cell DNA. Thus, the sealer should be biocompatible, inducing or enabling the bone repair. This biocompatibility is largely tested by implanting subcutaneous and sub osseous sealer samples 6,10,25.
In the studies of Kangarloo et al.. 11 and Scelza et al.. 21, the cytotoxicity of AH Plus sealer at the first 24 hours of contact with the culture medium was high, reducing as the days went by. According to Scelza et al.. 21, this occurs because of the decreasing in the concentration of leachable components over time.
On the other hand, Konjhodzic-Prcic et al.. 13 observed the increasing cytotoxicity of AH Plus sealer after 24, 48 hours, and 7 days, with a smaller number of viable cells. The authors did not explain why the cytotoxicity of AH Plus increased instead of decreasing. They only cited that the number of dead cells were within the limits of insignificance within the cytotoxicity scale. The study of Ehsani et al.. 8 had a shorter period than the study of Konjhodzic-Prcic et al.. 13, but with similar results, indicating the cytotoxicity increasing of AH Plus sealer over time. Ehsani et al.. 8 believed that this increasing occurred due to the volatilization of formaldehyde during the hot incubation or setting process of the AH Plus sealer.
The three clinical cases presented here showed chronic periapical lesion. The preparation, irrigation solutions, sealers were standardized, but the obturation techniques differed. The accidental extrusion of AH Plus sealer towards the periapex occurred after thermoplasticization (clinical cases #1 and #3) and lateral condensation techniques (clinical cases #2), despite of the tactile and radiographic proof of main cone locking, demonstrating the flow capacity of this sealer.
The study of Alonso et al.. 2 verified the flow capacity of Endofill and AH Plus sealers. AH Plus had the highest flow capacity. Sydney et al.. 22 tested these same materials through the vertical flow into millimetric glass plate, but Endofill had the greatest initial flow than AH Plus. Between the second and third hour, the AH Plus showed an abrupt displacement, passively flowing until the fifth hour.
Because AH Plus is a thixotropic fluid, this sealer undergoes transformation of its internal structure, which promotes the alteration of the flow speed, accounting for the abrupt flow, after certain time. In the sealer extrusion towards the periapex, the solubility directly influences on the body's response 22.
Generally, the sealers should have low solubility in contact to the tissue fluids because the releasing of the chemical compounds may irritate the periapical tissues. The small solubility of AH Plus sealer would contribute for the antibacterial action and local repair 4,5.
Tanomaru-Filho et al.. 23 tested the repair capacity of the periapical tissues after the induced extrusion of the sealer. Histologically, AH Plus showed satisfactory tissue response leading to repair. Sari and Duruturk 20 concluded that the AH Plus sealer extrusion did not prevent the periapical healing, but contributed for the delay in the repair period.
No clinical case showed pain after the extrusion. The clinical and radiographic following-up period revealed no pain and swelling. The third case showed the radiolucency decreasing of the apical lesion on the following-up radiograph due to the chemical and mechanical action of the preparation together with the bactericidal and low toxic action of the extruded sealer.
Conclusion
AH Plus sealer has adequate properties for an endodontic filling material. After extrusion, no further damage is seen to the periapical tissues due to low cytotoxicity. The necropulpectomy cases reported here, we observed no pain symptomatology immediately after the extrusion and after the clinical and radiographic following-up periods.
References
1. Akcay M, Arslan H, Durmus N, Mese M, Capar ID. Dentinal tubule penetration of AH Plus, iRoot SP, MTA Fillapex, and GuttaFlow Bioseal root canal sealers after different final irrigation procedures: a confocal microscopic study. Lasers Surg Med. 2016;48:70-6. [ Links ]
2. Alonso FS, Gomes CC, Freitas LF, Gomes IC, Pinto SS, Penina P. Análise comparativa do escoamento de dois cimentos endodônticos: Endofill e AH Plus. UFES Rev Odontol. 2005 Jan- Apr;7(1):48-54.
3. Batista RFC, Hidalgo MM, Hernandes L, Consolaro A, Velloso TRG, Cuman RKN et al.. Microscopic analysis of subcutaneous reactions to endodontic sealer implants in rats. J Biomed Mater Res A. 2007 Apr;81(1):171-7.
4. Borges AH, Dorileo MCGO, Villa RD, Borba AM, Semenoff TADV, Guedes AO et al.. Physicochemical properties and surfaces morphologies evaluation of MTA FillApex and AH Plus. The Scientific World Journal. 2014:1-6.
5. Cañadas PS, Berástegui E, Gaton-Hernández P, Silva LAB, Leite GA, Silva RS. Physicochemical properties and interfacial adaptation of root canal sealers. Braz Dent J. 2014;25(5):435-41.
6. Candeiro GTM, Moura-Netto C, D'Almeida-Couto RS, Azambuja-Junior N, Marques MM, Cai S et al.. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int Endod J. 2015 Sep.
7. Canedo LSD, Caballero AD, Carballo RF. Extrusión de cemento sellador endodóntico al espacio periapical. Revista de la Facultad de Ciencias de la Salud. 2011;8(1):88-92.
8. Ehsani M, Zabihi E, Gharouee H. A comparison between cytotoxicity induced by two resin based sealers (2Seal and AH Plus) in Saos-2 and MG- 63 cell lines. Int J Mol Cell Med. 2012;1(4):198- 202.
9. Farmakis ETR, Kontakiotis EG, Tseleni- Kotsovili A, Tsatsas VG. Comparative in vitro antibacterial activity of six root canal sealers against Enterococcus faecalis and Proteus vulgaris. J Investig Clin Dent. 2012 Nov;3(4):271-5.
10. Hiyasat AS, Tayyar M, Darmani H. Cytotoxicity evaluation of various resin based root canal sealers. Int Endod J. 2010 Feb;43(2):148-53.
11. Kangarloo A, Sattari M, Rabiee F, Dianat SO. Evaluation of cytotoxicity of different root canal sealers and their effect on cytokine production. Iran Endod J. 2009 Jan;4(1):31-4.
12. Khedmat S, Momen-Heravi F, Pishvaei M. A comparison of viscoelastic properties of three root canal sealers. J Dent (Tehran). 2013 March 2;10(2):147-54.
13. Konjhodzic-Prcic A, Gorduysus O, Kucukkaya S, Atila B, Muftuoglu S, Zeybek D. In vitro comparison of cytotoxicity of four root canal sealers on human gingival fibroblasts. Med Arh. 2015 Feb;69(1):24-7.
14. Marin-Bauza GA, Silva-Souza YTC, Cunha SA, Rached-Junior FJA, Bonetti-Filho I, Sousa- Neto MD et al.. Physicochemical properties of endodontic sealers of different bases. J Appl Oral Sci. 2012;20(4):455-61.
15. Ormiga F, Assis DF, Risso PA. Ability of three endodontic sealers to fill the root canal system in association with gutta-percha. Open Dent J. 2016;10:12-8.
16. Parirokh M, Forghani FR, Paseban HP, Asgary S, Askarifard S, Mahani SE. Cytotoxicity of two resin-based sealers and a fluoride varnish on human gingival fibroblasts. Iran Endod J. 2015;10(2):89-92.
17. Portela JVV, Cardoso RJA, Sousa CJA, Ying HH. Biocompatibility of the different portions of the content of AH Plus® sealer tubes through subcutaneous implantation. Dental Press Endod. 2011 Oct-Dec;1(3):56-64.
18. Reiss-Araujo C, Araújo SS, Baratto-Filho F, Reis LC, Fidel SR. Comparison of apical leakage of endodontic sealers AH Plus, Sealapex, Sealer 26 and Endofill through clearing teeth. RSBO. 2009;6(1):21-8.
19. Roth KA, Friedman S, Levesque CM, Basrani BR, Finer Y. Microbial biofilm proliferation within sealer-root dentin interfaces is affected by sealer type and aging period. J Endod. 2012 Sep;38(9):1253-6.
20. Sari S, Duruturk L. Radiographic evaluation of periapical healing of permanent teeth with periapical lesions after extrusion of AH Plus sealer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Sep;104(3):54-9.
21. Scelza MZ, Bobina J, Alves GG. Effect of time of extraction on the biocompatibility of endodontic sealers with primary human fibroblasts. Braz Oral Res. 2012 Sep-Oct;26(5):424-30.
22. Sydney GB, Ferreira M, Deonizio MDA, Leonardi DP, Batista A. Analysis of the flow profile of six endodontic cements. RGO. 2009 Jan- Mar;57(1):7-11.
23. Tanomaru-Filho M, Tanomaru JMG, Leonardo MR, Silva LAB. Periapical repair after root canal filling with different root canal sealers. Braz Dent J. 2009;20(5):389-95.
24. Viapiana R, Moinzadeh AT, Camilleri L, Wesselink PR, Tanomaru-Filho, Camilleri J. Porosity and sealing ability of root fillings with gutta-percha and BioRoot RCS or AH Plus sealers. Evaluation by three ex vivo methods. Int Endod J. 2016;49(8):774-82.
25. Wei Z, Bing P. Tissue reactions after subcutaneous and intraosseous implantation of iRoot SP, MTA and AH Plus. Dent Mater J. 2015;34(6):774-80.
26. Willershausen I, Callaway A, Briseño B, Willershausen B. In vitro analysis of the cytotoxicity and the antimicrobial effect of four endodontic sealers. Head Face Med. 2011 Aug;7:15.
 Corresponding author:
Corresponding author:
Karen Lys Hubbe
Rua: Harold Drummond de Carvalho, n. 216 – Cajuru
CEP 82970-340
Curitiba – PR – Brasil
E-mail: karenlys@hotmail.com
Received for publication: September 12, 2016
Accepted for publication: November 4, 2016