Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.14 no.1 Joinville Jan./Mar. 2017
Case Report Article
Central giant cell granuloma (CGCG) in childhood: surgical treatment by maintaining the tooth germs
Rafael Correia Cavalcante I; Paola Fernanda Cotait de Lucas Corso I; Tuany Rayra Pinto Lisboa Dias II; Eduarda Schramm II; Paulo Henrique Couto de Souza III; Nelson Luis Barbosa Rebellato IV; Delson João da Costa IV; Rafaela Scariot V
I Oral and Maxillofacial Surgery, Federal University of Parana – Curitiba – PR – Brazil
II Federal University of Parana – Curitiba – PR – Brazil
III Oral and Maxillofacial Surgery Department, Pontifical Catholic University of Parana – Curitiba – PR – Brazil
IV Oral and Maxillofacial Surgery Department, Federal University of Parana – Curitiba – PR – Brazil
V Oral and Maxillofacial Surgery Department, Positivo University – Curitiba – PR – Brazil
ABSTRACT
Introduction: Central Giant Cell Granuloma (CGCG) is a nonneoplastic benign process, of unknown etiology, more common in children and young adults. When aggressive, the lesion may result in considerable bone destruction and deformation. Oral and Maxillofacial surgery strongly depends on the nature of injury and it may vary from more conservative to more aggressive approach. Case report: The aim of the present study is to report and analyze, a giant cell central lesion in a 7-year-old patient on the right side of mandible body treated by surgical enucleation, curettage, and maintenance of the tooth germs. Discussion: In less aggressive lesions, curettage followed by radiographic monitoring is the most widely suggested treatment choice. However, the "gold standard" for aggressive and deforming lesions would be en-bloc resection with a safety margin. Most revisions show recurrence rates of 15 to 20%, thus clinical monitoring is necessary at least one year after the intervention. Conclusion: After 12 months, panoramic radiograph and computed tomography indicated new bone formation and no recurrence. In addition, good healing of soft tissues and correct eruption of the teeth #42, #43 and #44 were observed.
Keywords: Forensic Dentistry; child abuse; questionnaire.
Introduction
Central giant cell granuloma (CGCG) is considered a benign proliferative non-neoplastic process, of unknown etiology, more common in children and young adults aged less than 30 years (60% of cases) 2. Jaffe first described CGCG in 1953, as a "reparative fibrous dysplasia" of jaw bones. Studies suggest greater female involvement when compared to men (72.7% of cases in women) 7. CGCG is typically reported as a slow-growing, painless lesion of the jaw that may cause a mass effect on surrounding tissues 2,7.
Radiographically, CGCG has unilocular or multilocular radiolucent areas with irregular or relatively regular edges, having a predominance of small cases where the lesion is present in multiple loci in both the mandible and maxilla, and shows cortical bone expansion 21. Considering the location, the highest number of recorded injuries reports the lower jaw as preferred site (twice frequently in mandible when compared to maxilla) 20. Upon variation in biological behavior, CGCG is classified between aggressive and non-aggressive, according to the presence of symptoms, duration, root resorption and recurrence rate. Both radiologic and histologic characteristics are critical to determinate response to therapy and clinical behavior. Radiographic features of aggressive lesions include rapid growth, cortical thinning or perforation, size greater than 5 cm, recurrence after surgery, tooth displacement or resorption. In addition, histologically, aggressive lesions show a large area occupied by giant cells, great size, greater nucleolar organization and high expression of CD34 adhesion factors 9,27.
Martin et al. suggested that this lesion should be differentiated from brown tumor in association with hyperthyroidism. Because both diseases have identical clinical and histopathological features, PTH levels should be examined. No PTH deviation encourages us to determine CGCG diagnosis 24.
Treatment should be planned according to clinical signs and symptoms and can vary from simple curettage associated with conservative therapies to en-bloc resection 25. However, recurrences rates after surgical intervention are reported to be higher than 70%. To minimize surgical morbidity, especially in children, medical treatments acting on tumor proliferation have been proposed 12. However, there is no evidence suggesting any superiority for medical over surgical treatment 29.
Alternative and adjunctive CGCG treatments, such as intralesional steroid injections, are reported to be an effective alternative to surgery. Other studies, however, suggested a paradoxical effect, promoting growth of lesion in some patients. Calcitonin therapy, via intranasal sprays as well as subcutaneous injections, also has been used successfully in CGCG treatment. Calcitonin is a hormone produced by thyroid gland that inhibits bone resorption of multinucleated giant cells while lowering serum calcium levels and stimulating osteoblasts 26,29.
A relatively new CGCG treatment modality is the use of systemic interferon alfa. Interferon mechanism of action remains unclear; however, its effect might be related to its antiangiogenic effect on aggressive vascular components of the lesion 16. It was previously reported that interferon therapy alone has shown mixed results, since complete resolution to failure. However, the use of daily dose of interferon for an extended period together with conservative treatment has shown positive results and enabled preservation of teeth and other vital structures, which might have been sacrificed in traditional resections 16,19. This new therapy modality has different side effects and potentially serious toxicity, so its use has been restricted to patients whom conservative approaches have failed.
Treatment with denosumab has also been described in literature. Denosumab is a IgG2 human monoclonal antibody type which targets the RANK/RANKL system. RANKL is an essential mediator to function, formation, and osteoclast survival 30. RANKL inhibition leads to activity reduction of CGCG aggressive osteoclast function. Some previous studies reported denosumab utilization to treat CGCG in children, showing a volume reduction as well as an ossification of the lesion. Side effects, on the other hand, were reported to be headaches, dorsal and extremities pain 30,31.
Bisphosphonates had also been described to treat CGCG in childhood. Bisphosphonates are drugs used to treat osteoporosis due to inhibition of osteoclast activity. In 2009, Landesberg et al. and Chien et al. reported CGCG cases in children treated with Alendronate. It was observed in both cases a secondary reossification of the lesion and conservation of tooth germs, avoiding surgery and large debridement. Nevertheless, oral bisphosphonates have been reported to increase osteonecrosis risk mainly when associated with tooth extractions 8,18.
The most common surgical procedure to treat CGCG is the curettage or surgical en-bloc resection, also removing approximately 5 mm of adjacent healthy tissue 1,13,22,34. Due to high relapse rates, CGCG treatment is reported to be aggressive even in children, although an intermediate technique consisting in en-bloc resection with conservation of basilar board and/or posterior board have also been suggested 5. The present study treatment choice was enucleation and curettage of lesion with conservation of tooth germs.
Case report
Male patient, leukoderma, 7 years old, was referred to the Oral and Maxillofacial department of the Federal University of Parana (UFPR) with an important and visible facial asymmetry on the right side of the body region of mandible, with 8 months of evolution. Patient reported moderate pain in the region. Significant occlusal changes and swelling were observed. Permanent teeth were not erupted due to lesion interposition, blocking physiological eruption of affected teeth.
Cone beam tomography showed bone fenestrations as well as cortical expansion and perforation. Panoramic radiograph showed a well-described, unilocular lesion in intimate relationship with posterior tooth germ.
Surgical treatment was chosen, consisting of removing the lesion through enucleation. To try to prevent recurrences, the team decided to conduct this removal with safety margin through osteotomy and removal of healthy tissue adjacent to lesion. Since the patient was young, we opted for a more conservative approach, so there would be no loss of the teeth involved. The tooth germs (#43, #44 and #45) were maintained. Only the teeth #74 and #75 were extracted. Suture of the region was conducted using vicryl.
At 12-month follow-up appointment, new complementary exams, such as panoramic radiograph and cone beam computed tomography of head were taken. No relapses of the lesion were observed. Results of laboratory tests indicated bone formation on the site previously occupied by injury, and suggested an absence of recurrence. Good soft tissue healing and correct eruption of teeth #42, #43 and #44 was also observed.
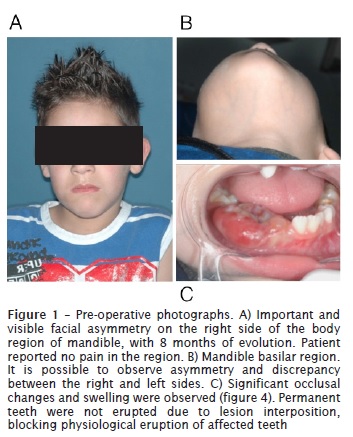
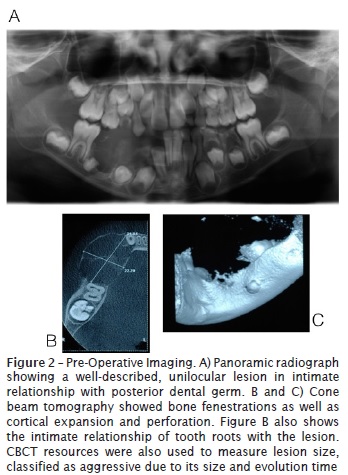
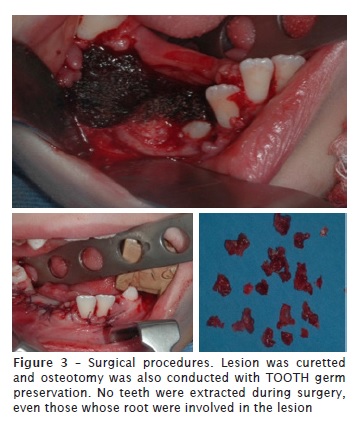
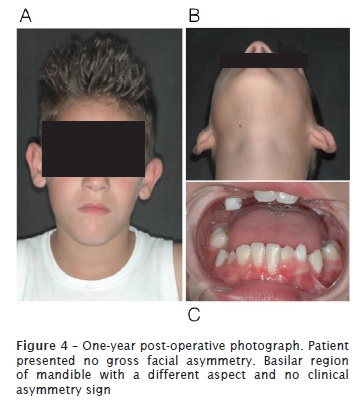
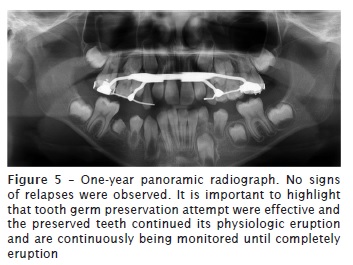
Discussion
Treatment modalities to central giant cell granuloma of the jaws are a dilemma, because it is hard to know which viable treatment is the best option for each patient. Although literature suggests higher CGCG prevalence in female (72.7% of cases) 33, this study reported a CGCG case in a young male patient of 7 years-old. Another decisive factor to determine the extent and predictability of treatment was the presence of tooth germs and the team's effort to not involve them in surgical treatment. The boy's legal responsible person was aware of all described methods currently used to treat the lesion.
Corticosteroid protocol to treat CGCG has shown varied results, from complete remission of lesion to tumor growth in others. No randomized controlled trials of this treatment have been conducted to determine its efficacy 6,31.
Even though different studies suggest success in isolated treatment with calcitonin therapy, there are others that indicate lack of response with intracutaneous injections and sometimes, progression of lesion after one-year treatment 25-27. The only randomized controlled trial on CGCG calcitonin treatment also showed no complete remissions 20. One possible reason why calcitonin therapy CGCG is effective for some lesions and not for others might rely on the fact that the expression of calcitonin receptors may vary from different populations. In one study, 41 specimens were analyzed and only 23 were reported to be positive to the receptor. A gold standard to determine whether calcitonin therapy would be effective includes immunohistochemical staining for calcitonin receptors protocols of biopsy specimens to determine the therapy course. We did not have our specimen stained, however it is expected that more surgeons and clinicians have the staining performed 32.
Treatment with interferon has shown positive results in multiple case reports. Most part of cases combines this therapy with curettage with success and complete lesion relapse 17. One case reported interferon therapy effective as monotherapy 10. Other studies however, reported a decrease in the size of the lesion but not total relapse. The theory that encourages interferon protocol utilization is that it may be more effective and beneficial to aggressive types of CGGCs, as interferon is an antiangiogenic medication and more aggressive CGCGs have a stronger ratio of vascularization when compared to indolent lesions 10,17. Although there are promising results in the use of interferon protocol to treat aggressive CGCGs, there are harmful side effects that must be considered before applying the use of this medication, such as bone marrow suppression and hair loss. In addition, interferon therapy is not used as first linage therapy it remains as savage procedure 14.
In June 2013, FDA approved using denosumab to treat CGCG in adults and some adolescents. According to FDA the intent of this therapy for those patients is to avoid severe morbidity of surgical procedures. Denosumab mechanism of action relies on inhibiting the receptor activator of nuclear factor kappa-B (RANK) and RANK ligand\osteoprotegerin (OPG) interaction, resulting in inhibition of osteoclast activity 30. Schreuder et al. reported a CGCG case of a young adult patient treated with Denosumab subcutaneous injections for 12 months after failing calcitonin and interferon therapies. They concluded that using Denosumad to treat aggressive CGCG lesions that did not respond to other therapies were effective, however, more studies are needed before it becomes a mainstay therapy. The most serious side effect of denosumab is medication-related jaw osteonecrosis and osteomyelitis 28. This has not been seen in the treatment of CGCG to date, but a thorough dental assessment before beginning of this medication regimen is recommended.
Surgery has always been considered the traditional treatment and it is still the most accepted one. On the other hand, some authors disagree on the surgery type performed as well as its indications 9. When teeth are associated with lesion, they are suggested to be retained if they do not compromise the removal of the lesion in question 3. CGCGs have traditionally been treated surgically. Common therapy is curettage or resection. Eisenbud et al advocated curettage or curettage plus peripheral osteotomy with maintenance of tooth germs for treatment of CGCG in children. He also suggested recurrence to range from 16% to 49%. Lesion relapses when they are removed via curettage were suggested to be treated with peripheral osteotomy and bone resection 1.
En-bloc resection might provide the greatest certainty of cure. Bataineh et al. conducted a study with 18 patients with aggressive CGCG, treated with en-bloc resection with 5 mm health tissue margin. Only one patient had lesion recurrence. Most authors agree that for child and growing patients, more conservative surgery is the only applicable strategy for maintaining the tooth germs to let them erupt physiologically 4. In general, destructive surgery (en-bloc surgical resection with 5 mm margins) seems to be the safest option for the control of recurrences but it may result in facial deformities, which are obviously of great concern. Due to aggressive characteristics observed in the patient reported in our case, we opted for conservative surgical treatment of CGCG through curettage and bone osteotomy, with success, without generate gross facial deformities and no relapse 12 months after surgery.
Conclusion
Different treatment modalities have been proposed to treat CGCG and each case must be analyzed differently regarding clinical features and lesion presentation, pathological behavior as well as patient conditions and limitations. CGCG in childhood must be looked with special attention, because invasive surgical procedures may cause facial deformities.
References
1. Aditya A, Aditya P. Central giant cell granuloma of jaw with multiple, multifocal recurrences. J Clin Diagn Res. 2016 Aug;10(8):3-4. [ Links ]
2. Barnes L, Eyeson J, Reichart P, Sjdransky D. Pathology and genetics of head and neck tumours. Lyon: IARC Pres; 2005.
3. Barthelemy I, Monday J. Tumeurs et pseudotumeurs des maxillaires riches en cellules geantes. Rev Stomatol Chir Maxillofac. 2009;110:209-13.
4. Bataineh A, Al-Khateeb T, Rawashdeh M. Tumeurs et pseudotumeurs des maxillaires riches en cellules geantes. J Oral Maxillofac Surg. 2002;60:756-61.
5. Bodner L. Cystic lesions of the jaws in children. Int J Pediatr Otorhinolaryngol. 2002;62:25-9.
6. Carlos R, Sedano H. Intralesional corticosteroids as an alternative treatment for central giant cell granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:161-70.
7. Chiba I, Teh G, Irzyka T, Fukada H. Conversion of a traumatic bone cyst into central mcell gigant granuloma. Implication for pathogenesis – a case report. J Oral Maxillofac Surg. 2002;60:222-5.
8. Chien M, Mascarenhas L, Hammoudeh J, Venkatramani R. Zoledronic acid for the treatment of children with refractory central giant cell granuloma. J Pediatr Hematol Oncol. 2015;37:399-401.
9. Chuong R, Kaban L, Kozakewich H, Perez-Atayde H. Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg. 1986 Sep;44(9):708-13.
10. Collins A. Experience with anti-angiogenic therapy of giant cell granuloma of facial bones. Ann R Australas Coll Dent Surg. 2000;15:170-5.
11. Dominguez-Caudrado L, Martinez-Gimeno C, Plasencia-Delgado J, Suner M. Intranasal calcitonin therapy for central giant cell granuloma. J Craniomaxillofac Surg. 2004;32:244-50.
12. Eisenbud L, Stern M, Rothberg M, Sachs S. Central giant cell granuloma of the jaws: experiences in the management of thirty – seven cases. J Oral Maxillofac Surg. 1988;46:376-80.
13. Ficarra G, Kaban L, Hansen L. Central giant cell lesions of the mandible and maxilla: a clinicopathologic and cytometric study. Oral Surg Oral Med Oral Pathol. 1987;64:44-50.
14. Goldman K, Marshall M, Alessandrini E, Bemstein M. Complications of alpha-interferon therapy for aggressive giant cell lesions of the maxilla. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:285-95.
15. Harris M. Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg. 1993;31:89-95.
16. Kaban K, Mulliken J, Ezekowitz R. Antiangiogenic therapy of a recurrent giant cell tumor of the mandible with interferon alpha-2a. Pediatrics. 1999;103:1145-7.
17. Kaban L. Biomedical technology revolution: opportunities and challenges for oral and maxillofacial surgeons. Int J Oral Maxillofac Surg. 2002;31:1-8.
18. Landesberg R, Eisig S, Funnoy I, Siris E. Alternative indications for bisphosphonate therapy. J Oral Maxillofac Surg. 2009;67:27-34.
19. de Lange J, van de Akker H, van de Berg H. Limited regression of central giant cell granuloma by interferon alpha after failed calcitonin therapy: a report of 2 cases. Int J Oral Maxillofac Surg. 2006;35:865-8.
20. de Lange J, van den Akker H, van de Berg H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Nov;104(5):603-15.
21. de Lange J, van Rijn R, van de Berg H, van den Akker H. Regression of central giant cell granuloma by a combination of imatinib and interferon: a case report. Br J Oral Maxillofac Surg. 2008 Jul;47:59-61.
22. O'Malley M, Pogrel MA, Stewart JC. Central giant cell granulomas of the jaws: Phenotype and proliferation-associated markers. J Oral Pathol Med. 1997;26:159-63.
23. O'Regan E, Gibbs D, Odell E. Rapid growth of giant cell granuloma in pregnancy treated with calcitonin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:532-40.
24. Martins W, Ribas M, Braosi A. Multiple giant cell lesions of the maxillofacial skeleton. J Oral Maxillofac Surg. 2007;65:1250-3.
25. Marx R, Sterm D. Oral and maxillofacial pathology: a rationale for diagnosis and treatment. Chicago: Quintessence Publishing; 2007.
26. Pogrel M, Regezi J, Harris S. Calcitonin treatment for central giant cell granulomas of the mandible: report of two cases. J Oral Maxillofac Surg. 1999;57:848-53.
27. Susarla S, Ausust M, Dewsnup N. CD34 staining density predicts giant cell tumor clinical behavior. J Oral Maxillofac Surg. 2009;67:951-6.
28. Schreuder W, Coumou A, Kessler P, de Lange J. Alternative pharmacologic therapy for aggressive central giant cell granuloma: denosumab. J Oral Maxillofac Surg. 2014;72:1301-9.
29. Terry B, Jacoway J. Management of central giant cell lesions. An alternative to surgical therapy. Oral Maxillofacial Surg Clin N Am. 1994;6:464-8.
30. Thomas D, Henshaw R, Skubitz K, Chawla S, Station A, Blay J. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275-80.
31. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant cell tumour of bone. Lancet Oncol. 2010;11:815-7.
32. Vered M, Buchner A, Dayan D. Immunohistochemical expression of glucocorticoid and calcitonin receptors as a tool for selecting therapeutic approach in central giant cell granuloma of the jaw bones. Int J Oral Maxillofac Surg. 2006;35:756-60.
33. Waldron CA, Shafer WG. The central giant cell reparative granuloma of the jaws. An analysis of 38 cases. Am J Clin Pathol. 1966;45(4):437-47.
34. Whitaker SB, Waldron CA. Central giant cell lesions of the jaws. Oral Surg Oral Med Oral Pathol. 1993;75:199-201.
 Corresponding author:
Corresponding author:
Rafael Correia Cavalcante
Rua Professor Lothario Meissner, 623 – Jardim Botânico
CEP 80210-170 – Curitiba
Paraná – Brasil
E-mail: rafaelcorreia14@gmail.com
Received for publication: November 1, 2016
Accepted for publication: December 19, 2016













