Services on Demand
Article
Related links
Share
Arquivos em Odontologia
Print version ISSN 1516-0939
Arq. Odontol. vol.48 n.3 Belo Horizonte Jul./Sep. 2012
ARTIGO ORIGINAL
In vitro evaluation of cytotoxicity of different brands of artificial teeth
Avaliação in vitro da citotoxicidade de dentes artificiais de diferentes marcas comerciais
João Milton Rocha GusmãoI; Matheus Melo PithonI; Rogerio Lacerda dos SantosII; Maria Teresa Villele RomanosIII
IDepartment of Health, School of Dentistry, Universidade Estadual do Sudoeste da Bahia (UESB), Jequié, BA, Brazil
IIDepartment of Health and Rural Technology, Universidade Estadual da Paraíba (UEPB), Patos, PB, Brazil
IIIProfessor Paulo Goes Institute of Microbiology, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil
ABSTRACT
Aim: The present study aimed to evaluate the cytotoxicity of different brands of stock acrylic teeth against fibroblast cells.
Materials and Methods: For this experiment, the cell lineage used was the L929 from the American Type Culture Collection (ATCC, Rockville, MD, USA). The brands of stock teeth tested included: Artplus, Biolux, Biotone, Pop Dent, Vipi Dent, Orthosit Ivoclar, Biocler, and Trilux. Each group was placed in contact with the fibroblast cells for the periods of 24, 48, 72, and 168 hours. Control cells, as well as negative and positive controls, were used to compare the extremes.
Results: The results showed cytotoxicity for all the groups tested in the time periods of 24, 48, and 72 hours during the experiment, which occurred in the Orthosit Ivoclar and Trilux groups even after the experiment had been conducted for 168 hours.
Conclusion: The researched acrylic teeth developed cytotoxicity against the cells in vitro during the time periods of 24, 48, and 72 hours, while Ivoclar Vivodent and Trilux brands of teeth developed cytotoxicity against the tested cells during the time period of 168 hours.
Uniterms: Tooth artificial. Evaluation studies. Cell line.
RESUMO
Objetivo: O objetivo deste estudo foi avaliar a citotoxicidade de diferentes marcas de dentes de estoque contra células fibroblásticas.
Materiais e Métodos: Para esta experiência a linhagem celular utilizada foi L 929 American Type Culture Collection (ATCC, Rockville, MD). As marcas de dentes de estoque testadas foram: Artplus, Biolux, Biotone, Pop Dent, Vipi Dent, Orthosit Ivoclar, Biocler Trilux. Cada grupo foi colocado em contacto com as células fibroblasticas nos períodos de 24, 48, 72 e 168 horas. Controles positivo e negativo foram usados para comparação com os extremos.
Resultados: Os resultados mostraram citotoxicidade para todos os grupos testados nos períodos de 24, 48 e 72 horas da realização do experimento, o que ocorreu para os Grupos Orthosit Ivoclar e Trilux ainda após 168 horas de condução do experimento.
Conclusão: Os dentes de acrílico estudados pesquisadas desenvolveram citotoxicidade contra as células fibroblásticas in vitro, nos períodos de 24, 48 e 72 horas e Vivodent Ivoclar e Trilux desenvolveram citotoxicidade contra as células testadas até o período de 168 horas.
Descritores: Dente artificial. Estudos de avaliação. Linhagem celular.
INTRODUCTION
Similar to that occurring in other countries, the Brazilian population is undergoing a process of aging in the age pyramid. A significant portion of the elderly population is partially or completely edentulous, which can be explained by the absence of preventive health policies geared toward the adult population in Brazil, coupled with inadequate restorative-surgical dental treatments that are far more mutilating than those aimed at maintaining teeth1.
The dental materials used to manufacture dental prostheses commonly stay in contact with biological structures when functioning in the oral cavity, acting as foreign bodies2 capable of causing alterations in the oral cavity tissues3. For this reason, it is necessary to test the possible toxic effect of these dental materials on cells.
Since the 1930s, acrylic resins have been used to manufacture dental prostheses4; however, biocompatibility has generally represented the main factor limiting their use5. The majority of total and partial removable dentures are manufactured using stock acrylic teeth, the main advantage of which is a firm chemical bond to the denture base acrylic resin, in such a way that there is no need to manufacturemechanical retainers5-11. A wide range of commercial brands of teeth are available on the dental product market, which have different aesthetic and functional characteristics, and are mainly chosen in accordance with the patient’s financial situation.
Therefore the present study aimed to evaluate the cytotoxicity of different brands of stock acrylic teeth against L929 fibroblast cells.
MATERIALS AND METHODS
The cell lineage used in this experiment was the L929 (mouse fibroblast) from the American Type Culture Collection (ATCC, Rockville, MD, USA), cultivated in minimum essential medium Eagle (MEM) (Cultilab, Campinas, São Paulo, Brazil) and supplemented with 2mM of L-glutamin (Sigma, St. Louis, Missouri, USA), 50mg/ml of gentamicin (Schering Plough, Kenilworth, New Jersey, USA), 2.5mg/ml of fungizone (Bristol-Myers-Squibb, New York, USA), 0.25ml of sodium bicarbonate solution (Merck, Darmstadt, Germany), 10mM of HEPES (Sigma, St. Louis, Missouri, USA), and 10% Fetal bovine serum (FBS) (Cultilab, Campinas, São Paulo, Brazil). This cell lineage was kept at 37ºC in an environment containing 5% CO2.
To perform this experiment, eight different types of stock teeth were used, divided into eight groups, denominated as follows: Artiplus (Dentsply, Rio de Janeiro, Brazil), Biolux (Vipi, São Paulo, Brazil), Biotone (Dentsply, Rio de Janeiro, Brazil), Pop dent (DentBras, São Paulo, Brazil), Vip Dent (Vipi, São Paulo, Brazil), Orthosit Ivoclar (Ivoclar Vivadent, Schaan, Liechtenstein), Biocler (DentBras, São Paulo, Brazil), and Trilux (Vipi, São Paulo, Brazil).
To verify the cell response against the extremes, another three groups were inserted into the evaluations: Group CC (cell control), in which the cells were not exposed to any material; Group C+ (positive control), consisting of one amalgam test specimen; and Group C- (negative control), consisting of stainless steel wire, all of which remained in contact with the cells.
The materials were sterilized prior to exposure to UV light (Labconco, Kansas, Missouri, USA) for 1 hour. After, three samples of each material were placed in 24-well plates containing culture medium (MEM) (Cultilab, Campinas, São Paulo, Brazil). After every 24h time period, the culture medium was replaced with a new medium. The supernatants were collected after 24, 48, 72, and 168 hours and were evaluated with regard to toxicity against L929 cells. The supernatants were placed in triplicate in a 96-well plate containing a confluent monolayer of L929 cells and incubated for 24 hours at 37ºC in an environment containing 5% CO2, totaling nine wells to be evaluated (n=9). After the incubation time, the effect on cell viability was determined by means of the dye-uptake technique, described by Neyndorff et al.12, with slight modifications.
After 24 hours of incubation, 100ml of 0.01% neutral red (Sigma, St. Louis, Missouri, USA) was added to the culture medium in each well of the miniplates and was incubated at 37oC for 3 hours for the dye to penetrate into the live cells. After this time interval had elapsed, and after discarding the dye, 100ml of 4% formaldehyde solution (Reagen) was added to the PBS (NaCl 130 mM; KCl 2 mM; Na2HPO4 2H2O 6mM; K2HPO4 1mM, pH7.2) for 5 minutes, to promote cell fixation to the plates. Next, to extract the dye, 100ml of 1% acetic acid solution (Vetec, Rio de Janeiro, Brazil) with 50% methanol was added (Reagen, Rio de Janeiro, Brazil). After 20 minutes, the reading was measured by a spectrophotometer (BioTek, Winooski, Vermont, USA) at a wavelength of 492nm (l = 492 nm).
Statistical analyses were performed using the SPSS 13.0 program (SPSS Inc., Chicago, Illinois, USA). Descriptive statistical analyses, including mean and standard deviation, were calculated for the evaluated groups. The values for the quantity of viable cells were first submitted to an analysis of variance (ANOVA) to determine if there was any statistical difference among the groups. After, the Tukey test was performed.
RESULTS
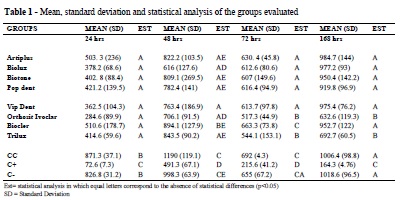
After 24h of incubation, no statistical difference could be observed regarding the number of viable cells for the tested groups of teeth. By contrast, the extremes did present a statistical difference among the groups, while the cell control and negative control presented no statistical difference between them. After 48 hours, Artplus, Biotone, Pop Dent, and Trilux presented similar results when the groups were compared. A similarity in results also occurred when comparing the Biotone with the Orthosit Ivoclar groups. However, when comparing the Vip Dent with the Biocler groups, different results could be observed. Statistical difference could be identified among all the tested groups when compared to the control groups. After 72 hours, the Artiplus, Biolux, Biotone, Pop Dent, and Vip Dent groups presented no statistical difference among them; the Orthosit Ivoclar and the Trilux groups presented statistically similar results; while the Biocler group presented results that were different from all the other tested groups. Results from the tested groups were statistically different from those of the control groups. After 168 hours, the Artiplus, Biolux, Biotone, Pop Dent, Vip Dent, and Biocler groups presented statistically similar results and presented no statistical difference when compared to the cell control and the negative control. The Trilux and Orthosit Ivoclar groups, although similar to one another, presented a statistically significant difference when compared to the other groups and controls. The positive control proved to be statistically different from all the groups (Table 1).
The results of the experiment showed that during the time periods of 24, 48, and 72 hours, all the tested groups of teeth presented statistically different results from those of the cell control and negative control. In addition, based on the number of viable cells, a cytotoxic effect on the tested cells could be observed. In the time period of 168 hours, only the Trilux and Orthosit Ivoclar groups still developed cytotoxic effects on the tested cells.
DISCUSSION
Dentures made of acrylic resin, or which contain this resin in their composition, are widely used, such as: conventional complete dentures, partial removable dentures with clips or retained by connections, fixed implant supported dentures (Branemark protocols), and implant retained removable dentures (overdentures). As these artificial structures remain in constant contact with an individual’s oral cavity cells, which may occur throughout one’s life, it is thus pertinent to evaluate the possible toxic effects on cells developed by acrylic resin.
Acrylic resins used to manufacture artificial teeth for prosthetic purposes are similar to those used to make complete denture bases. The difference that exists is the much higher number of cross links present in the material that the teeth are made of, making them apt to perform the function for which they are intended, namely incision and food grinding13.
Despite the subject’s importance, no studies evaluating the cytotoxicity of stock teeth made of acrylic resin can be found in the literature, which makes this study relevant from a scientific point of view. The studies from the literature with closely related methodologies have studied acrylic resin for complete denture and partial removable denture bases14-22. The majority of these studies present unfavorable results regarding the behavior of acrylic resin, which develops a cytotoxicity against the tested cells.
In the present study, during the three initial periods of the experiment, a toxic effect on the fibroblast cells could be identified for all the groups of teeth tested. Shin and Watts19 observed that after a prolonged polymerization cycle of the denture base acrylic resin, a reduction in the amount of residual monomer released could be observed. Based on this result, it is possible to conclude that residual monomer released by the groups of teeth had occurred in the three initial periods of the experiment.
Moreover, within the limits of this study, the results allow us to suppose that the acrylic resin constituent of the majority of tested teeth had no relevant cytotoxic effect on the cells, since only the Orthosit Ivoclar and Trilux groups, among the eight tested groups, still presented cytotoxicity in the time period of 168 hours and even thereafter. However, it was not possible to affirm whether or not the cytotoxic effect remained for an undetermined period of time in the Orthosit Ivoclar and Trilux groups. Therefore, further studies are warranted concerning these groups.
CONCLUSION
In the light of this experiment, it could be concluded that:
The researched acrylic teeth did in fact develop a cytotoxicity against the cells in vitro in the time periods of 24, 48, and 72 hours during the experiment, and only the Orthosit Ivoclar and Trilux brands of teeth developed cytotoxicity against the tested cells in the time period of 168 hours.
REFERÊNCIAS
1. Kiyak HA, Mulligan K. Studies of the relationship between oral health and psychological wellbeing. Gerodontics. 1987; 3:109-12. [ Links ]
2. Tang ATH, Li J, Ekstrand J, Liu Y. Cytotoxicity tests of in situ polymerized resins: methodological comparisons and introduction of a tissue culture insert as a testing deice. J Biomed Mater Res. 1999; 45:214-22. [ Links ]
3. Park JB. Biomaterials. In: Bronzino JD, editor. Biomedical engineering handbook. Boca Raton: CRC Press and IEEE Press; 1995. p.530–610.
4. Beall JR. Wear of acrylic resin teeth. J Am Dent Assoc. 1943; 30:252-56. [ Links ]
5. Appelbaum M. Theories of posterior tooth selection: porcelain versus acrylic. Dent Clin North Am. 1984; 28:299-306. [ Links ]
6. Craig RG, O’Brien WJ, Powers JM. Plásticos em Prótese. In: Craig RG, O’Brien WJ, Powers JM, editors. Materiais dentários: propriedades e manipulação. Rio de Janeiro: Guanabara Koogan; 1988. p.169-84.
7. Winkler S, Monasky GE, Kwok J. Laboratory wear investigation of resin posterior denture teeth. J Prosthet Dent. 1992; 67:812-4. [ Links ]
8. Hirano S, May KB, Wagner WC, Haker CH. In vitro wear of resin denture teeth. J Prosthet Dent. 1998; 2:152-5. [ Links ]
9. Kawano F, Ohguri T, Ichikawa T, Mizuno I, Hasegawa A. Shock absorbability and hardness of commercially available denture teeth. Int J Prosthodont. 2002; 15:243-7. [ Links ]
10. Neyndorff HC, Bartel DL, Tufaro F, Levy JG. Development of a model to demonstrate photosensitizer-mediated viral inactivation in blood. Transfusion. 1990; 30:485-90. [ Links ]
11. Anusavice, KJ. Phillips materiais dentários. 11nd ed. Rio de Janeiro: Elsevier; 2005. [ Links ]
12. Harrison A, Huggett R. Effect of the curing cycle on residual monomer levels of acrylic resin denture base polymers. J Dent. 1992; 20:370-4. [ Links ]
13. Lefebvre CA, Knoernschild KL, Schuster GS. Cytotoxicity of eluates from light-polymerized denture base resins. J Prosthet Dent. 1994; 72:644-50. [ Links ]
14. Schuster GS, Lefebvre CA, Dirksen TR, Knoernschild KL, Caughman GB. Relationships between denture base resin cytotoxicity and cell lipid metabolism. Int J Prosthodont. 1995; 8:580-6. [ Links ]
15. Tsuchiya H, Hoshino Y, Tajima K, Takagi N. Leaching and cytotoxicity of formaldehyde and methyl methacrylate from acrylic resin denture base materials. J Prosthet Dent. 1994; 71:618-24. [ Links ]
16. Wennberg A. Biological evaluation of root canal antiseptics using in vitro and in vivo methods. Scand J Dent Res. 1980; 88:46-52. [ Links ]
17. Campanha NH, Pavarina AC, Giampaolo ET, Machado AL, Carlos IZ, Vergani CE. Cytotoxicity of hard chairside reline resins: effect of microwave irradiation and water bath postpolymerization treatments. Int J Prosthodont. 2006; 19:195-201. [ Links ]
18. Jorge JH, Giampaolo ET, Vergani CE, Pavarina AC, Machado AL, Carlos IZ. Effect of microwave postpolymerization treatment and of storage time in water on the cytotoxicity of denture base and reline acrylic resins. Quintessence Int. 2009; 40:93-100. [ Links ]
19. Shim JS, Watts DC. Residual monomer concentrations in denture-base acrylic resin after an additional, soft-liner, heat-cure cycle. Dent Mater. 1999; 15:296-300. [ Links ]
20. Sheridan PJ, Koka S, Ewoldsen NO, Lefebvre CA, Lavin MT. Cytotoxicity of denture base resins. Int J Prosthodont. 1997; 10:73-7. [ Links ]
21. Vallittu PK, Miettinen V, Alakuijala P. Residual monomer content and its release into water from denture base materials. Dent Mater. 1995; 11:338-42. [ Links ]
22. Bagis YH, Rueggeberg FA. The effect of postcure heating on residual, unreacted monomer in a commercial resin composite. Dent Mater. 2000; 16:244-7. [ Links ]
 Autor correspondente:
Autor correspondente:
João Milton Rocha Gusmão
Departmento de Saúde, Faculdade de Odontologia
Universidade Estadual do Sudoeste da Bahia
Av. José Moreira Sobrinho, S/N, Jequiezinho
CEP: 45.206-19 – Jequié – Bahia – Brazil
e-mail:joao.milton@ig.com.br
Recebido em 16/04/2012 – Aceito em 30/05/2012