Services on Demand
Article
Related links
Share
RFO UPF
Print version ISSN 1413-4012
RFO UPF vol.17 n.2 Passo Fundo May./Aug. 2012
Immunohistochemical assessment of the MIB, CK-14, p63, p16, Cal A, and Cys A markers in spindle cell squamous cell carcinomas of the lip
Avaliação imuno-histoquímica dos marcadores MIB, CK-14, p63, p16, Cal A e Cys em carcinomas espinocelulares de lábio
Marília Gerhardt de OliveiraI ; Patrícia W. Fregapani WormII; Juliana Dreyer da Silva de MenezesIII; Luciana Maria Pereira RamalhoIV; João Feliz Duarte de MoraesV; Ferdinando De ContoVI
I Full Professor, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, Brazil.
II Oral and Maxillofacial Surgeon, PUCRS - Porto Alegre - Brazil
III Dentist, PUCRS, Porto Alegre, Brazil
IV Professor, School of Dentistry, UFBA, Salvador, Brazil
V Professor, School of Mathemathics, UFRGS, Porto Alegre, Brazil
VI Professor, School of Dentistry, UPF, Passo Fundo, Brazil
ABSTRACT
Objective: with the objective of testing the expression of the protein markers MIB, CK14, p63, p16, Cal A, and Cys A in the pathogenesis of oral spindle cell carcinoma, we conducted an immunohistochemical study of the expression of the protein markers MIB, CK14, p63, p16, Cal A, and Cys A in human biopsy specimens of these lesions. Methods: fifteen histological specimens of spindle cell squamous cell carcinoma of the lower lip were obtained from the Department of Oral Pathology, Bahia Federal University. Immunohistochemical analyses were performed at the Molecular Biology Laboratory of the Department of Otorhinolaryngology, Heidelberg University, Germany. Results: statistical analysis revealed no association between markers. There was strong positive staining for Ck14, MIB, and Cal A in 93.3% of cases, thus establishing a strong association. Conclusion: p63, p16, MIB, Cal A, Cys A are markedly expressed and p16 is strongly suppressed in oral cavity tumors, which suggests that the latter protein may play a role in negative regulation of cell cycle progression.
Keywords: Diagnostic. Oral cancer.
RESUMO
Objetivo: O objetivo deste estudo foi avaliar, através de iomunohistoquímica, a expressão dos marcadores protéicos MIB, CK14, p63, p16, Cal A e Cys A em lesões bucais com diagnóstico histopatológico de carcinoma espinocelular. Métodos: 15 amostras histológicas com diagnóstico confirmado localizado em lábio inferior obtidas do Serviço de Patologia Bucal da Universidade Federal da Bahia foram examinadas no Laboratório de Biologia Molecular do Departamento de Otorrinola Otorrinolaringologia da Universidade de Heidelberg, Alemanha. Resultados: O estudo estatístico não revelou associação entre os marcadores. Os genes Ck14, MIB e Cal A apresentaram intensa marcação nas células do tecido, em 93,3% dos casos, estabelecendo assim, forte relação. Conclusão: Os resultados suportam a investigação revelando que os genes MIG, CK14, p63, Cal A e Cys A se apresentam fortemente evidentes nos tumores de cavidade oral e o p16 suprimido, sugerindo que esta proteína pode exercer um papel de regulador negativo do ciclo celular.
Palavras-chave: Cãncer bucal. Diagnóstico. Imuno-histoquímica.
Introduction
Immunohistochemical identification of molecular genetic events in the progression of preneoplastic lesions to spindle cell squamous-cell carcinoma enables early detection of lesions with the potential for malignant progression, thus permitting timely intervention1,2.
The various markers that enable assessment of the progression of preneoplastic lesions to spindle cell carcinoma include the p16 protein, which halts the cell cycle and induces apoptosis by pRb-mediated phosphorylation of cyclin-dependent kinase 4 (CDK4). Functional loss of p16 may lead to uncontrolled cell proliferation3,4. Another protein, calgranulin A (Cal A), is involved in the regulation of several cell processes, including the cell cycle and cell differentiation. Its expression has recently been associated with the development of severe dysplasias5-8. Cystatin A (Cys A), a cysteine protease inhibitor, is a precursor of proteins involves in keratinocyte keratinization, and is expressed during the late phase of differentiation of these cells. Studies suggest that expression of cystatin A is inversely associated with malignant progression of cancer9.
Other markers, such as retinoblastoma and p53, may be related with early steps of carcinogenesis in oral cavity squamous cell carcinoma. Furthermore, higher Rb expression has also been observed in malignant lesions10. The p63 protein, a homologue of p53, may be associated with tumor formation in the epithelial tissue, acting as an oncogene11,12. Expression of p63 is almost exclusively restricted to epithelial cells, mutations in this gene are infrequent, and its expression is increased in a variety of solid tumors, particularly those of the head and neck area12,13. MIB (Ki-67) is one of the cell cycle regulator proteins that is found during duplication, being higher in carcinomas than in hyperplasias, indicating poor prognosis14,15. CK14 is positive in basaloid squamous cell carcinoma, and is mostly distributed diffusely in the basaloid cells; it is a parameter of proliferative activity and metastatic potential of squamous cell carcinoma of the lung16,17.
Methods
Sample selection
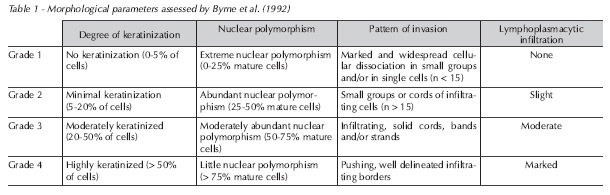
Fifteen histological specimens of spindle cell squamous cell carcinoma of the lower lip were obtained from the Department of Oral Pathology, Bahia Federal University. Preliminary histological analysis with H&E staining was performed to grade lesions according to degree of keratinization, nuclear polymorphism, pattern of invasion, and lymphoplasmacytic infiltrate, using the system developed by Byrne et al. (1992)2 (Table 1). Changes were scored on a scale of 1 to 4 points according to intensity, and scores were added to yield a mean lesion grade for each slide.
Morphological parameters
Immunohistochemistry
After biopsy selection, immunohistochemical analyses were performed at the Molecular Biology Laboratory of the Department of Otorhinolaryngology, Heidelberg University, Germany.
Specimens were sliced into 4 μm sections, yielding one slide for each tested antibody. After removal of paraffin by xylol immersion and rehydration in a graded alcohol series and deionized water, antigen recovery was performed for the MIB, CK14, p63, p16, Cal A, and Cys A proteins. Sections were placed in citrate buffer (10 mmol/L), microwaved for 3 minutes at 700W and 10 minutes at 200W, left to cool at room temperature, and rinsed with deionized water and PBS. After inactivation of endogenous peroxidase and soaking in horse serum for 1 hour, sections were incubated in primary anti-MIB, anti-CK14, anti-p63, anti-p16, anti-Cal A, and anti-Cys A antibodies.
Criteria for positive immunohistochemical staining.
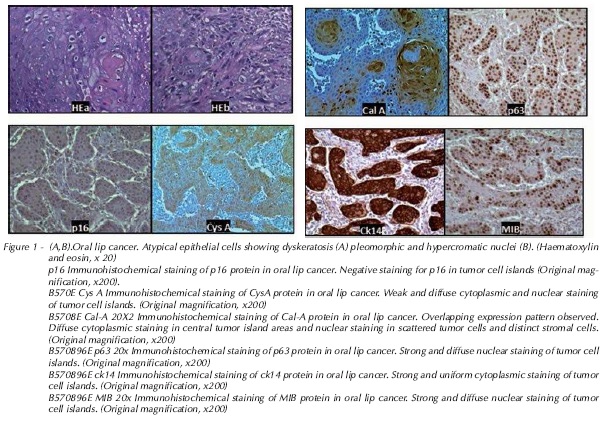
Immunohistochemistry findings were analyzed semiquantitatively. Expression was considered absent (-) in slides with ≤ 10% positive cells; moderate (+) in those with 10-50% positive cells; and marked (++) in those with ≥ 50% positive cells. Preliminary findings showed differences in expression of the MIB, CK-14, p63, p16, Cal A, and Cys A markers.
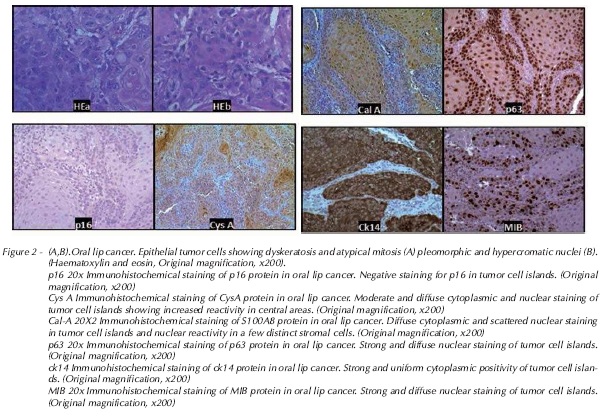
Results
Descriptive statistics were used to assess the immunohistochemical expression of CK-14, MIB, p63, Cys A, Cal A, and p16 in lower lip spindle cell carcinoma specimens. Fisher's exact test was used to tabulate data.
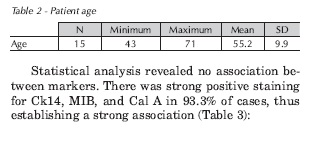
All patients were male. Mean age was 55 (range, 43-71 years) (Table 2).
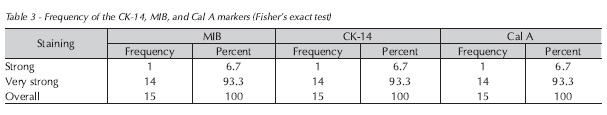
Statistical analysis revealed no association between markers. There was strong positive staining for Ck14, MIB, and Cal A in 93.3% of cases, thus establishing a strong association Table 3.
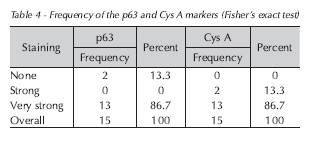
There was strong, positive tissue staining for the p63 and Cys A genes in 86.7% of cases, as shown in Table 4.
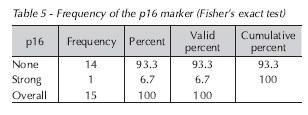
There was no positive association with the p16 gene, as staining for this marker was absent in 93.3% of cases (Table 5):
Discussion
To overcome this challenge and gain a better understanding of cell survival and apoptosis, cell proliferation and tumor suppression markers have been studied in the search for more reliable indicators for detection and prediction of the progression of preneoplastic or malignant lesions18-21. A better understanding of the molecular mechanisms that involve control of neoplasm cell growth may enable identification of the factors involved in tumor regulation and progression. From the simple count of mitoses, which provides generic evidence2,22, to the regulatory mechanisms of the cell cycle, the relationship between growth factors, oncogenes, tumor suppressor genes, and their protein products plays an important role in the detection and quantification of proliferating cells1. Therefore, biomarkers are relevant to the clinical management of patients with malignant neoplasms, as they assist in diagnosis, staging, assessment of treatment response, detection of recurrence, and prognostication. Despite their major clinical application, there is little scientific evidence in this area23,24, which justifies further research, such as this study.
The possibility of analyzing mRNA from the archives of pathology laboratories is exciting, as it allows for large retrospective studies. Formalin is the most common fixative used in the surgical pathology routine, and its promotion of nucleic acid degradation is well known. In addition, the proteins extracted from formalin-fixed and paraffin-embedded tissue seem to be the same compared with those extracted from fresh frozen tissue. Additionally, easier- to-use immunohistochemical markers of apoptosis, applicable in archived paraffin-embedded tissue, have been commercially developed.25-27
Among the various markers reported to be positive in premalignant and malignant lesions, p16 stands out21. This study evaluated the p16 protein due to its important role in the pathogenesis of head and neck cancer28, as cell cycle progression depends on the action of cyclin-CDK complexes. This process is inhibited by a group of proteins that modulate the action of these complexes, known as cyclin-CDK complex inhibitors (CKIs)29, which includes p16. Functional loss of this gene may be associated with development and progression of a variety of malignant neoplasms30. Under normal conditions of controlled cell division, p16 acts as a negative regulator of the cell cycle, which may predispose to occurrence of the initial steps required for malignization28. In this study, there was no expression of p16 in 93% of cases, which confirms the hypothesis that p16 is a tumor suppressor gene.
The p63 protein, a p53 homologue, may be associated with tumor formation in epithelial tissues, thus acting as an oncogene11,12. There has been substantial recent interest in the role of p63 as a regulator of cell proliferation and differentiation in potentially and frankly malignant lesions. As expression of p63 is almost exclusively restricted to epithelial cells, mutations are infrequent, and its expression is increased in a variety of solid tumors, particularly those of the head and neck area12,13. In our sample, approximately 86% of specimens were strongly positive on immunohistochemical staining, which is consistent with the literature. Even more markedly, MIB (Ki-67) and CK-14 were equally expressed (93%) in our specimens, corroborating previous studies that reported a worse prognosis in tumors that exhibit affinity for this marker14,15,31.
Cys A expression has been reported in polymorphonuclear granulocytes, keratinocytes, and liver and spleen tissue32. This protein plays an important role in inhibition of apoptosis caused by caspase-3 inhibition, giving the cell more time to promote DNA repair33. In this study, Cys A was detected very often (86.7%) in specimens of lower lip cancer, which suggests an association with tumor pathogenesis.
The Cal A protein is closely related to regulation of intracellular activities such as abnormal growth, cell cycle progression, transcription, and cellular differentiation6. The Cal A gene has been the object of particular research attention due to its involvement in a variety of human diseases, such as rheumatoid arthritis, acute inflammation, cardiomyopathies, Alzheimer's disease, and cancer13. Expression of this protein in normal epithelial tissue, including the oral mucosa, is well established. The role of Cal A in neoplasms is still unclear, but appears to be closely tied to the differentiation potential of epithelial tissue, as it is strongly expressed in keratinocyte hyperproliferation. Dysregulation of this gene has been described in epithelial cell carcinoma of the head and neck, as in a study that revealed strong expression in nasopharyngeal tumors5.
Dysregulation of S100 expression may be associated with development of tumors and metastases6. Furthermore, protein expression studies have detected increased levels of S100-like proteins, such as Cal A, in a variety of tumor tissues as compared to their corresponding abundance in normal tissue13. This is consistent with the data reported in this study, in which approximately 94% of samples exhibited strong immunohistochemical staining for this protein, which suggests it is closely related to spindle cell carcinoma.
Conclusion
The results of this study support investigation of the relationship between cell cycle biomarkers and the pathogenesis of oral cancer. Particularly relevant is the finding that p63, p16, MIB, Cal A, Cys A are markedly expressed and p16 is strongly suppressed in oral cavity tumors, which suggests that the latter protein may play a role in negative regulation of cell cycle progression.
Acknowledgements
This work was supported by the National Council for Scientific and Technological Development (CNPq), Brazil; and Heidelberg University, Germany.
References
1. Tahara E. Genetic alteration in human gastrointestinal cancers: the application to molecular diagnosis. Cancer 1995; 75(6):1410-7. [ Links ]
2. Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol 1992; 166: 375-81.
3. Wang JL, Zheng BY, Li XD, Angstrom T, Lindstrom MS, Wallin KL. Predictive significance of the alterations of p16INK4a, p14ARF, p53, and proliferating cell nuclear expression in the progression of cervical cancer. Clin Cancer Res 2004; 10: 2407-14.
4. Schlecht NF, Trevisan A, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL, et al. Viral load as a predictor of the risk of cervical intraepithelial neoplasia. Int J Cancer 2003; 103: 519-24.
5. Fung LF, Lo AKF, Yuen PW, Liu Y, Wang XH, Tsao SW. Differential gene expression in nasopharyngeal carcinoma cells Life Sciences 2000; 67: 931-6.
6. Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci 1996; 21:134-40.
7. Kong CS, Balzer BL, Troxell ML, Patterson BK, Longacre TA. P16INK4a immunohistochemistry is superior to HPV in situ hybridization for the detection of high-risk HPV in atypical squamous metaplasia. Am J Surg Pathol 2007; 31: 33-43.
8. Lesnikova I, Lidang M, Hamilton-Dutoit S, Koch J: p16 as a diagnosticmarker of cervical neoplasia: a tissue microarray study of 796 archivalspecimens. Diagn Pathol 2009, 4:22.
9. Holladay EB, Logan S, Arnold J, Knessel B, Smith DG. A comparison of the clinical utility of p16INK4a imunolocalization with the presence of human papillomavirus by hybrid capture 2 for the detection of cervical dysplasia/neoplasia. Cancer 2006; 108:451-61
10. Oliveira MG, Piazza T, Gaiao L, Ramalho LMP, Pozza DH, De Mello RA. Retinoblastoma and p53 protein expression in pre-malignant oral lesions and oral squamous cell carcinoma. Mol Med Report 2012. In Press
11. Bérnard J, Douc-Rasy S, Ahomadegbe C. TP53 family members and human cancers. Human Mut 2003; 21:182-91.
12. Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death inducing and dominant- negative activities. Mol Cell 1998; 2:305-16.
13. Melle C, Ernst G, Schimmel B, Bleul A, Koscielny S, Wiesner A et al. A Technical Triade for Proteomic Identification and Characterization of Cancer Biomarkers. Cancer Res 2004; 64:4099-104.
14. Aaltomaa S, Lipponen P, Vasalainen S, Ala-Opas M, Eskelinen M, Syrjanen K. Value of Ki-67 immuno labelling as a prognostic factor in prostate cancer. Eur Urol 1997; 32:410-5.
15. Madani SH, Ameli S, Khazaei S, Kanani M, Izadi B. Frequency of Ki-67 (MIB-1) and P53 expressions among patients with prostate cancer. Indian J Pathol Microbiol 2011; 54(4):688-91.
16. Winters R, Naud S, Evans MF, Trotman W, Kasznica P, Elhosseiny A. Ber-EP4, CK1, CK7 and CK14 are useful markers for basaloid squamous carcinoma: a study of 45 cases. Head Neck Pathol 2008; 2(4):265-71. Epub 2008 Oct 19.
17. Tsubokawa F, Nishisaka T, Takeshima Y, Inai K. Heterogeneity of expression of cytokeratin subtypes in squamous cell carcinoma of the lung: with special reference to CK14 overexpression in cancer of high-proliferative and lymphogenous metastatic potential. Pathol Int 2002; 52(4):286-93.
18. MacPhee DG. Mismatch repair, somatic mutations, and the origins of cancer. Cancer Res 1995; 55(23):5489-92.
19. Sudbo J, Reith A. Which putatively pre-malignant oral lesions become oral cancers? Clinical relevance of early targeting of high-risk individuals. J Oral Pathol Med 2003; 32: 63-70.
20. Klaes R, Benner A, Friedrich T, Ridder R, Herrington S, Jenkins D et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Sur Pathol 2002; 26:1389-99.
21. Serrano M, Hannon GJ, Beach D. A new regulatory motif incell-cycle control causing specific inhibition of cyclin D/ CDK4. Nature 1993; 366: 704-7.
22. Cheng KC, Loeb LA. Genomic instability and tumor progression: mechanistic considerations. Adv Cancer Res 1993; 60:121-56.
23. Akrish S, Buchner A, Dayan D. Oral cancer: diagnostic options as an aid to histology in order to predict patients at high risk for malignant transformation. Refuat Happen Vehashinayin 2004; 21(4):6-15.
24. Sdubo J, Samuelsson R, Risberg B et al. Risk markers of oral cancer in clinically normal mucosa as an aid in smoking cessation counseling. J Clin Oncol 2005; 20; 23(9):1927-33.
25. Leite KRM, Canavez JMS, Reis ST, Tomiyama AH, Piantino CB, Sañudo A et al. miRNA analysis of prostate cancer by quantitative real time PCR: Comparison between formalinfixed paraffin embedded and fresh-frozen tissue. Urol Oncol 2011; 29(5): 533-7.
26. Gräntzdörffer I, Yumlu S, Gioeva Z, von Wasielewski R, Ebert MPA, Röcken C. Comparison of different tissue sampling methods for protein extraction from formalin-fixed and paraffin-embedded tissue specimens. Exp Mol Pathol 2010; 88(1):190-6.
27. Holubec H, Payne CM, Bernstein H, Dvorakova K, Bernstein C, Waltmire CN et al. Assessment of Apoptosis by Immunohistochemical Markers Compared to Cellular Morphology in Ex Vivo-stressed Colonic Mucosa J Histochem Cytochem 2005; 53(2):229-35.
28. Ohta S, Uemura H, Matsui Y, Ishiguro H, Fujinami K, Kondo K et al. Alterations of p16 and p14ARF genes and their 9p21 locus in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107:81-91.
29. Chen QM, Luo G, Li BQ, Samaranayake LP. Expression of p16 and CDK4 in oral premalignant lesions and oral squamous cell carcinoma: a semi-quantitative immunohistochemical study. J Oral Pathol Med 1999; 28:158-64.
30. Akanura D, Uzawa N, Yoshida MA, Negishi A, Amagasa T,Ikeuchi T. Inactivation patterns of the p16 (INK4a) gene in oral squamous cell carcinoma cell lines. Oral Oncol 1999; 35:476-83.
31. Gasparoni A, Fonzi L, Schneider GB, Wertz PW, Johnson GK, Squier CA. Comparison of differentiation markers between normal and two squamous cell carcinoma cell lines in culture. Arch Oral Biol 2004; 49: 653-64.
32. Westerink WMA, Stevenson JCR, Horbach GJ, Schoonen WGEJ. The development of RAD51C, Cystatin A, p53 and Nrf2 luciferase-reporter assays in metabolically competent HepG2 cells for the assessment of mechanism-based genotoxicity and of oxidative stress in the early research phase of drug development. Mutat Res 2010; 696: 21-40.
33. Takahashi H, Komatsu N, Ibe M, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Cystatin A suppresses ultraviolet B-induced apoptosis of keratinocytes, J Dermatol Sci 2007; 46: 179-187.
 Address for Correspondence:
Address for Correspondence:
Ferdinando De Conto
Rua Teixeira Soares, 1075 sala 1002 Edifício Tamandaré
99010-080 - Passo Fundo-RS
e-mail: ferdi@upf.br
Recebido: 26/04/2012
Aceito: 01/08/2012