Services on Demand
Article
Related links
Share
Brazilian Journal of Oral Sciences
On-line version ISSN 1677-3225
Braz. J. Oral Sci. vol.10 n.2 Piracicaba Apr./Jun. 2011
ORIGINAL ARTICLE
Salivary characteristics of diabetic children
Gheena S.I; Chandrasekhar T.II; Pratibha RamaniIII
I MDS, Senior Lecturer, Department of Oral and Maxillofacial Pathology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai, India
II MDS, Professor and Head of Department, Department of Oral and Maxillofacial Pathology, Saveetha Dental College, Saveetha University, Chennai, India
III MDS, Professor, Department of Oral and Maxillofacial Pathology, Saveetha Dental College, Saveetha University, Chennai, India
ABSTRACT
Aim: The objectives of this study were to evaluate the levels of glucose, cholesterol, protein and albumin in saliva, and to correlate the levels of glucose of the saliva to oral health and blood glucose of diabetic and non-diabetic children. Methods: 32 children with type 1 diabetes mellitus formed the study group (DC) and 32 non-diabetic children formed the control group (ND). The patients had their saliva collected and evaluated for glucose, cholesterol, total protein and albumin. Blood glucose analysis was also performed. The dental health status of the subjects was measured by DMFT index and def index. Independent Student’s t-test was performed to compare metabolic status values in DC and ND groups. Correlation test was applied between blood glucose and salivary glucose (Spearman’s correlation), and salivary glucose and DMFT/def (Spearman’s test). Results: A statistically significant difference was observed between DC and ND considering salivary glucose (p=0.000). Elevated levels of cholesterol were evident in DC in correlation with ND. Total protein and albumin had increased values in DC (nonsignificant p value). The dental health status was not statistically different. Conclusions: Salivary parameters can act as adjuncts in assessing the overall metabolic status of the patient.
Keywords: saliva, diabetes, pediatric, glucose, cholesterol.
Introduction
Diabetes mellitus (DM) comprises a group of common metabolic disorders that share the salient feature of hyperglycemia. Several distinct types of DM exist and are caused by a complex interaction of genetics, environmental factors and lifestyle choices. The metabolic deregulation associated with DM causes secondary pathophysiological changes in multiple organ systems and imposes a tremendous burden on the individual with diabetes and the health care system.
Although all forms of DM are characterized by hyperglycemia, the pathogenic mechanisms by which hyperglycemia arises differ widely. The two broad categories of DM are designated as type1 (IDDM) and type 2 (NIDDM). The incidence of childhood onset type 1 diabetes is increasing in many countries in the world, at least in the under 15-year-old age group. There are strong indications of geographic differences in trends but the overall annual increase is estimated to be around 3%.Two international collaborative projects, the Diabetes Mondiale study (DiaMond)1 and the Europe and Diabetes study (EURODIAB)2 have been instrumental in monitoring trends in incidence.
By 2010, it is estimated that annually some 76,000 children aged less than 15 years develop type 1 DM worldwide. Of the estimated 480,000 children with type 1 DM, 24% come from the South-East Asian Region, but the European Region, where the most reliable and up-to-date estimates of incidence are available, comes a close second (23%)3.The incidence rate of type 1 DM in India is 4.2/100,000 population per year.
A wide spectrum of oral manifestations of DM has been reported ranging from xerostomia, taste impairment, sialosis, dental caries and periodontal disease to fungal infections, oral lichen planus and fissured tongue4. However, in children, there is no agreement in results that relate alterations of salivary chemical composition and oral health5.
An optimum metabolic control level correlates positively with the well being of the patient. Assessing the metabolic control of the patient has traditionally been done through testing of glycosylated hemoglobin, fructosamine, and glycoalbumin along with other criteria such as Body Mass Index, microalbuminuria and dyslipidemia6-7. Fructosamine reflects the glycemic control over the previous 1-2 weeks and has been correlated positively with serum albumin and serum total protein5. Saliva is a unique fluid and interest in it as a diagnostic medium has advanced exponentially in the last decade. Advances in technology have helped to move saliva beyond evaluating oral health characteristics to where it now may be used to measure essential features of overall health8.
Thus, the objectives of this study were to evaluate the levels of glucose, cholesterol, protein and albumin in saliva, and to correlate the levels of glucose of the saliva to oral health and blood glucose of diabetic and non-diabetic children thereby analyzing whether saliva-based glucose testing can be an effective alternative to blood-based glucose testing in children.
Material and methods
Study population
The study group comprised 32 diabetic children (DC) aged between 5 and 15 years with an already established diagnosis of type 1 DM. This group was selected from the patients attending the outpatient diabetes department, Institute of child health and research centre, Egmore, Chennai. The control group comprised 32 non-diabetic children (ND) without any other pre-existing systemic diseases. The study was conducted in collaboration with the Institute of Child Health and Research Centre and College of Dental Surgery, Saveetha University, Chennai with the ethical committee approval [(ihep no.) MDS 34/SU 63/06]. Parental informed consent was obtained for all patients before they were examined and samples collected.Saliva collection and processing
Sample collection was performed during the morning hours when the subjects are in the fasting mode. Additionally, blood glucose values determined from venous blood samples drawn at the time of collection of saliva (fasting blood sugar) were also taken into account.
Standard UCLA procedure was used to collect the saliva. The subjects were asked to refrain from eating, drinking or other oral hygiene procedures, for at least one hour prior to collection. Drinking water was then given to the subjects, to rinse their mouth. Five minutes after the oral rinse, unstimulated saliva was collected in 50 mL falcon tubes by the method of spitting the saliva. The patient was asked to swallow the saliva present in the mouth and then to remain still without moving the tongue or swallowing the saliva for one minute. The patient spat the saliva every 60 s for a total of 5 min into a falcon tube to which sodium fluoride9 has been added. The tubes were then placed on ice. About 1.5 mL of saliva was collected from each subject. The samples were then centrifuged at 2,500 rpm for 5 min. The centrifugation resulted in a saliva sample which is free of large particulate debris and reduced viscosity, thereby allowing a more accurate and reproducible analysis.
Determination of glucose
Glucose was estimated by using a glucose kit (CREST BIOSYSTEMS, A division of Coral Clinical Systems, Goa, India) based on glucose oxidase-peroxidase method. It was standardized for saliva with 0.384mg/dL - as the lower value. The absorbance of the standard and the test material (0.01 mL/10 μL) were measured against the blank without dilution within 60 min at a wavelength of 505 nm (Hg 546 nm)/ Green. Total glucose in milligram/deciliter (mg/dL) is then derived as Absorbance of test (Abs.T)/ Absorbance of standard (Abs.S) X 100.
Determination of Cholesterol
Cholesterol (CH) was estimated by using a commercially available cholesterol kit, which is based on cholesterol oxidase /PAP method. The absorbance of the standard and the test material (0.01 mL/10μL) without dilution were measured against the blank within 60 min at a wavelength of505nm (Hg 546nm)/ Green. Cholesterol in mg/dL = Abs.T/ Abs. S X 200.
Determination of Total Protein
Total protein (TP) was estimated by using a commercially available test kit based on the biuret method. The absorbance of the standard and the test material (0.02 mL/20 μL) are measured against the blank without dilution within 60 min at a wavelength of 550 nm (Hg 546 nm)/ yellow – green. Total proteins in gram (g)/dL = Abs.T/Abs.S x8.
Determination of Albumin
Estimation of albumin (ALB) was done by the colorimetric method based on the BCG method (commercially available test kits). The absorbance of the standard and the test material (0.01 mL/10 μL) are measured without dilution against the blank within 60 min at a wavelength of 630 nm (Hg623nm)/ Red. Albumin in g/dL= Abs.T/Abs.S x 4.
Dental health status
The dental health status of the subjects was measured by the DMF-T index and the def index. The DMF index pertains to the permanent teeth (D – Decayed, M- Missing and F – Filled) and the def index (d – decayed, e – extracted, f – filled) to the deciduous teeth.

Statistical analysis
Independent Students’t-test was performed to compare metabolic status values in DC and ND groups. Correlation test was applied between blood glucose and salivary glucose (Spearman’s correlation), and salivary glucose and DMFT/ def (Spearman’s test).
Results
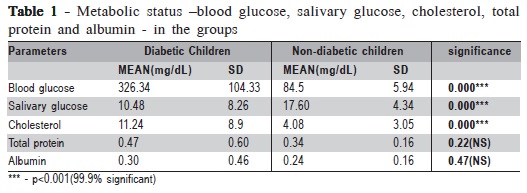
The mean value of salivary glucose (SG) of DC is 10.4 mg/dL and for ND is 17.6 mg/dL. The resulting p = 0.000 was 99.9% significant. A statistically significant difference (p = 0.000) was found between DC and ND with regard to cholesterol values. No statistically significant difference was found between DC and ND with regard to albumin (p = 0.22) and total protein (p = 0.47) (Table 1).
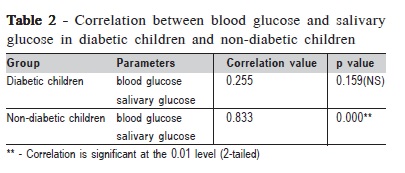
There was no significant correlation between salivary glucose (SG) and blood glucose (BG) within DC, but there was a significant correlation within ND (p=0.000) (Table 2). The correlation between the DMFT status and the salivary glucose values of the subjects yielded nonsignificant results
Discussion
Whole saliva is a mixture of the secretions produced by the three pairs of large glands and the smaller glands of oral mucosa (labial, lingual, buccal and palatal). It may also contain fluid from the gingival pocket (gingival, or crevicular fluid)9. The serum constituents in saliva are derived from the local vasculature of the salivary glands as well as from gingival fluid. The increased salivary glucose evident in the whole saliva can be due to several contributing factors or it could be a simple reflection of the blood glucose levels since saliva is an ultra filtrate of plasma. The salivary analytes are derived from plasma generally by three mechanisms (passive diffusion, active transport and ultra filtration) and are thus found in saliva as mentioned by Miller10.
A chronic disease like DM, with its emphasis on patient involvement in the control of the disease, and its attendant
problems, place a huge burden on the pediatric patients. Our efforts should be directed at making it an easier burden to bear. If saliva could be used for diagnosis and monitoring, diabetic children would not require daily invasive tests, they can just collect their saliva in a sterilized collection tube, which could then be used in biosensors for the real-time, sensitive and specific detection of salivary diagnostic analytes to give an overall view of the child’s diabetic status. Very little information is available on the potential of saliva in diabetic children in India.
The salivary glucose values of the case subjects in our study were lower than the controls. This finding is similar to those of previous studies11-13. Reuterving et al.11 determined whether salivary flow rate and salivary glucose concentration in patients with diabetes mellitus influence of severity of diabetes. Salivary glucose concentration was lower during the period of better metabolic control. Marchetti et al.12 found that the salivary glucose secretion rate was significantly lower (p less than 0.02) in diabetic patients with diabetic autonomic neuropathy than normal patients. Kanji et al.13 measured the plasma and salivary concentrations of glucose and cortisol during insulin induced hypoglycemic stress in healthy Nigerians. Salivary glucose levels (fasting and after intravenous insulin) were unaffected by hypoglycemia and did not correlate with plasma glucose at any time point.
We could not ascertain the reason for the low salivary glucose concentrations evident in DC in comparison to ND. One of the confounding variables may be that the cases in DC were under treatment as opposed to the cases in ND. More studies are needed on this facet.
The salivary concentration of Lipid soluble, unconjugated steroids such as cortisol, estriol, testosterone and progesterone, closely reflects the plasma concentration. The significantly high values of salivary cholesterol seen in the diabetic subjects could be an indication of the dyslipidemia, a
condition usually associated with diabetic subjects14-15.
Dyslipidemia is a common feature of DM and needs to be treated because of the potential complications especially atherosclerosis. Cholesterol being a lipid moiety passes into saliva by passive diffusion and has the potential to closely reflect blood concentrations.
The cholesterol of case subjects is statistically higher than that for the control subjects. Karjailanen et al.16 suggested that salivary cholesterol may be regarded as a transudate from serum, as suggested by Slomiany et al. The positive correlation of serum and saliva cholesterol values further supported the concept that at least part of the salivary cholesterol originates from serum. They also concluded that, in healthy adults, salivary cholesterol concentrations reflect serum concentration to some extent and can be used to select individuals with high serum cholesterol levels. Medical Laboratory Observer (2000) gives information about a salivabased cholesterol test that has demonstrated the potential to correlate a quantitative result with patient blood cholesterol level.
Ben Aryeh et al.17 found no difference in salivary total protein, amylase, lactoferrin, or lysozyme among the three groups (IDDM, NIDDM, Controls) examined. Streckfus et al.18 recorded that all diabetic groups in their study demonstrated a significantly lower salivary total protein concentration when compared with controls, which is in contrast to our study values.
There is a lack of literature on total protein and albumin estimations from the saliva of diabetic subjects, and the available data do not show a consensus on the results. These varied results indicate, as suggested by Rantonen et al.19 that these proteins are subject to short-term variation. Improved ways of evaluation of these elements in saliva with sophisticated techniques or another way of assessing the metabolic control in saliva needs to be considered.
The diabetic group and the control group did not differ with regard to the dental status as opposed to the usual scenario where diabetic children are expected to have more carious lesions. This could be attributed to the reduction in the frequency of sticky food intake and food with high glycemic index and better oral hygiene practices by the diabetic children. Swanljung et al.20 found that if the patients’ IDDM is well controlled, their salivary and caries data does not differ from that of healthy controls. Blanco et al.21 found no differences in the number of decayed, missing and filled teeth based on metabolic control, evolution time and existence of late complications of diabetes.
The lack of correlation between the salivary glucose and blood glucose values in DC underscores the importance of more studies needed in this direction. Both saliva and blood samples were taken during morning hours when the case subjects were in fasting mode. One of the confounding variables may be the fact that the cases in DC were under treatment as opposed to the cases in ND.
The cholesterol in DC was significantly higher than that of ND. Further research is needed, especially on the correlation of salivary and blood values of cholesterol, to have a better understanding of this relationship.
Total protein and albumin can be used to assess the metabolic status of diabetic subjects since both showed a slight increase as compared to controls. The dental caries experience correlation between the cases and controls was not significant and gives credence to the value of diet and oral hygiene measures in the subjects. Salivary parameters can act as adjuncts in assessing the overall metabolic status of the patient. Further studies should be performed to make these tests equivalent to a blood test.
Acknowledgements
The authors express their sincere gratitude to Dr. N. Malathi, Faculty of Dental Sciences, SRU; Director, Institute of Child Health; Dr. Parivardhini, Diabetes OPD, Institute of Child Health; Mr. Porchelvan and Mr. Ravi Shanker, statisticians, Sri Ramachandra Medical College and Research institute for their help and motivation.
References
1. DIAMOND. Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006; 23: 857-66. [ Links ]
2. Patterson CC. Dahlquist GG. Gyürüs E. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet.2009; 373: 2027-33.
3. International Diabetes Federation. The Diabetes Atlas. 4th ed. Montreal: International Diabetes Federation; 2009.
4. Manfredi M, Mc Cullough MJ, Vescovi P, Al-Kaarawi ZM, Porter SR. Update on Diabetes Mellitus and related oral diseases. Oral Dis. 2004; 10: 187-200.
5. Lopez ME, Colloca ME, Paez RG, Schallmach JN, Koss MA, Chervonagura A. Salivary characteristics of diabetic children. Braz Dent J. 2003; 14: 26-31.
6. Kaplan L, Pesce A. Clinical Chemistry theory, analysis, and correlation. Saint Louis: Mosby; 1984.
7. The Clinical Chemistry of Diabetes, Workshop, Washington, DC, Oct 1998.
8. Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002; 8: 69-76.
9. Jenkins GN. The physiology and biochemistry of the mouth. Oxford: Blackwell; 1978. v.4.
10. Miller SM. Saliva: new interest in a non-traditional specimen. Saliva as a multipurpose diagnostic fluid. Med Lab Observ. 1993; 20: 31-5.
11. Reuterving CO, Reuterving G, Hagg E, Ericson T. Salivary flow rate and salivary glucose concentration in patients with diabetes mellitus influence of severity of diabetes. Diabete Metab. 1987; 13: 457-62.
12. Marchetti P, Tognarelli M, Giannarelli R, Grossi C, Picaro L, di Carlo A et al. Decreased salivary glucose secretory rate: usefulness for detection of diabetic patients with autonomic neuropathy. Diabetes Res Clin Pract. 1989; 7: 181-6.
13. Alkanji AO, Ezenwaka C, Adejuwon CA, Osotimehin BO. Plasma and salivary concentrations of glucose and cortisol during insulininduced hypoglycaemic stress in healthy Nigerians. Afr J Med Med Sci. 1990; 19: 265-9.
14. Ladeia AM, Adan L, Couto-Silva AC, Hiltner A, Guimaraes AC. Lipid profile correlates with glycemic control in young patients with type 1 diabetes mellitus. Prev Cardiol. 2006; 9: 82-8.
15. Wiltshire EJ, Hirte C, Couper JJ. Dietary fats do not contribute to hyperlipidemia in children and adolescents with type 1 diabetes. Diabetes care. 2003; 26: 1356-61.
16. Karjalainen S, Sewon L, Soderling E, Larsson B, Johansson I, Simell O et al. Salivary cholesterol of healthy adults in relation to serum cholesterol concentration and oral health. J Dent Res. 1997; 76: 1637-43.
17. Ben-Aryeh H, Serouya R, Kanter Y, Szargel R, Laufer D. Oral health and salivary composition in diabetic patients. J Diabetes Complications. 1993; 7: 57-62.
18. Streckfus CF, Marcus S, Welsh S, Brown RS, Peppers GC, Brown RH. Parotid function and composition of parotid saliva among elderly edentulous African-American diabetics. J Oral Pathol Med. 1994; 23: 277-9.
19. Rantonen PJ, Meurman JH. Correlations between total protein, lysozyme, immunoglobulins, amylase, and albumin in stimulated whole saliva during daytime. Acta Odontol Scand. 2000; 58: 160-5.
20. Swanljung O, Meurman JH, Torkko H, Sandholm L, Kaprio E, Maenpaa J. Caries and saliva in 12-18-year-old diabetics and controls. Scand J Dent Res. 1992; 100: 310-3.
21. Arrieta - Blanco JJ, Villar BB, Martinez EJ,Vallejo PS, Arrieta - Blanco FJ. Bucco-dental problems in patients with Diabetes Mellitus (I) : Index of plaque and dental caries. Med Oral. 2003; 8: 97-109.
 Correspondence:
Correspondence:
Gheena.S
Dept of Oral and Maxillofacial Pathology,
Faculty of Dental Sciences, Sri Ramachandra
University, Porur, Chennai, India
Phone: +91 - 98840 33777
E-mail: gheena_ranjith@yahoo.co.in
Received for publication: July 15, 2010
Accepted: May 31, 2011