Services on Demand
Article
Related links
Share
Brazilian Journal of Oral Sciences
On-line version ISSN 1677-3225
Braz. J. Oral Sci. vol.10 n.2 Piracicaba Apr./Jun. 2011
ORIGINAL ARTICLE
Evaluation of the polylactide-polyglycolide copolymer Fisiograft® in the treatment of intrabony defects. Clinical and radiographic results of a pilot study
Abhinav Bansal.I; Shobha Prakash.II
I MDS, Senior Lecturer, Department of Periodontics, Peoples College of Dental Sciences and Research Centre, Bhopal, Madhya Pradesh, India
II BDS, MDS , Professor & H.O.D, Department of Periodontics, College of Dental Sciences, Davangere, Karnataka, India
ABSTRACT
Aim: To evaluate the efficacy of the polylactide-polyglycolide copolymer Fisiograft® as a bone graft material in the treatment of interproximal intrabony defects clinically and radiographically. Methods: A total of 22 intrabony defects in 8 patients with chronic periodontitis (4 males and 4 females) aged 20 to 55 years were recruited and divided equally into two groups: control (open flap debridement alone) and experimental (open flap debridement with Fisiograft®). Recordings of probing pocket depth (PPD), clinical attachment level (CAL), gingival margin position (GMP) and radiovisiographic assessment was done at baseline and 6 months. Results: Statistical analysis was done by Wilcoxon Signed Rank test for intra-group comparisons and Mann-Whitney U-test for inter-group comparisons. The clinical parameters PPD, CAL and GMP were found to be statistically significant (p<0.05) within each group. Inter-group comparison showed only the CAL gain to be statistically significant (p<0.01). In relation to hard tissue changes, statistically significant (p=0.05) result was seen for the percent filling of the original defect, comparing the experimental and control groups 6 months postoperatively. Conclusions: Placement of Fisiograft® resulted in better healing of intrabony defects as assessed clinically and radiographically when compared to open flap debridement alone.
Keywords: Fisiograft®, intrabony defects, open flap debridement, randomized clinical trial.
Introduction
The reconstruction or restoration of osseous defects caused by inflammatory periodontal disease is a continuing challenge in periodontal therapy. The immediate goal in the treatment of periodontal disease is to arrest and control or eliminate periodontal disease and prevent its recurrence. However, the ultimate goal of periodontal therapy is to restore the structure, integrity, and function of tissues that have been lost as a result of inflammatory periodontal disease1.
Periodontal therapies such as scaling, root planning and gingival curettage, are highly effective in reducing the likelihood of periodontal disease progression, but they have an extremely limited capacity to stimulate regeneration. Among treatment modalities, grafting of biomaterials/bone substitutes have been used with different success rates for the reconstruction of the lost periodontal attachment. The first use of bone grafts in the periodontal therapy is credited to Hegedus2. Histologic evidence in humans indicates that bone grafting is the only treatment that leads to regeneration of bone, cementum, and functionally oriented new periodontal ligament coronal to the base of previous osseous defect1.
Biodegradable polymers, especially those belonging to the family of polylactic acid (PLA) and polyglycolic acid (PGA), have played an increasingly important role in bone re-constructive procedures. Although extensively used in orthopedics for over a decade, PLA/PGA biomaterials have been scarcely used in craniomaxillofacial applications. In dentistry, surgical sutures and absorbable membranes in PGA and/or PLA acids have been available for some time for use in guided tissue regeneration3-4. However, only in recent years absorbable synthetic biopolymers have been used as bone fillers in periodontics, proving effective stimulants to bone regeneration5-6. Implantation of PLA-derived devices has been studied to prevent alveolar osteitis or dry socket in extraction sites7-8 as well as in the treatment of periodontal osseous defects with access flap alone, PLA implant and decalcified freeze-dried bone allograft 9.
Recently, a new copolymer (Fisiograft®) , Ghimas SpA, Casalecchio di Reno, Italy) of 50% DL lactic acid and 50% glycolic acid (50 PLA: 50 PGA) mixed with dextran 125 as excipient, has been marketed in different formulations, such as sponge, gel, and powder. Being synthetic, it is absolutely free from risk of cross contamination due to pathological agents such as bovine spongioform encephalopathy (BSE), hepatitis and human immunodeficiency virus (HIV). It is biocompatible and well tolerated as it is reabsorbed and degraded in Kreb’s cycle. It can be easily molded, shaped and function as an absorbable space maintainer. This material has lower molecular weight, which permits a more rapid biological degradation, being completely absorbed within 4-6 months. Fisiograft® has osteoconductive properties because it is penetrated and totally substituted by trabecular bone. Animal studies have shown that Fisiograft® is an osteoconductive degradable copolymer that results in formation of new bone matrix and trabeculae10. Another study in post-extraction sockets in mini-pigs has shown that, after 30 days of Fisiograft® placement, the material was degraded and the sockets were filled with bone, which, in terms of bone volume fraction, trabecular number, and separation, was not statistically different from normal bone11. Fisiograft® has recently been used to enhance bone formation in human extraction socket12 as well as in maxillary sinus augmentation procedures13-14. In a pilot study to evaluate the healing of large defects in human jaw bone, the clinical and histologic performance of Fisiograft® was found to be between that of autologous bone and platelet rich plasma.
Fisiograft® showed some lamellar formation and delayed regenerative capability without requiring second donor site15. Fisiograft® has recently been used in the treatment of deep periodontal intraosseous defects with variable results. In combination with autogenous bone graft, Fisiograft® has been proven effective in the treatment of dehiscence defect around immediate implants16.
Since few clinical studies have assessed the effect of polylactide-polyglycolide copolymer (Fisiograft®) in the treatment of deep periodontal intraosseous defects, this study was done to evaluate the clinical and radiographic outcomes of reconstructive surgery in human intrabony defects with use of Fisiograft® in combination with open flap debridement (OFD) procedure as compared to OFD alone.
Material and methods
Study population
Eight patients (4 males and 4 females) with chronic periodontitis in the age range of 20-55 years were considered for this study. Patients having at least two interproximal periodontal pockets measuring >= 5 mm with radiographic evidence of vertical bone loss (3-wall defect, which was confirmed at the time of surgery) were selected from the outpatient section of the Department of Periodontics, College of Dental Sciences, Davangere, Karnataka, India. Pregnant and lactating females, smokers or tobacco chewers, patients who had any systemic disease or allergy, or those who had received any form of periodontal therapy within 6 months prior to the study were excluded. The purpose and design of the clinical trial was explained and informed consent was obtained from the prospective study participants. The study protocol was approved by the Research Ethics Committee of College of Dental Sciences, Davangere, Karnataka, India.
Prior to surgery, a total of 22 sites were divided into two groups: 11 sites were randomly assigned to the experimental group and were treated with OFD plus Fisiograft®, and the other 11 sites were identified as control sites and treated with OFD alone.
Presurgical procedure
After an initial examination and treatment planning discussion, all selected patients were given detailed instructions on self-performed plaque control measures and were subjected to phase-I periodontal therapy. Four weeks after phase-I therapy, the oral hygiene status and the tissue response was evaluated using plaque index (PI)17 and gingival index (GI)18 and the patients were subjected to the surgical procedure.
Clinical and radiographic measurements
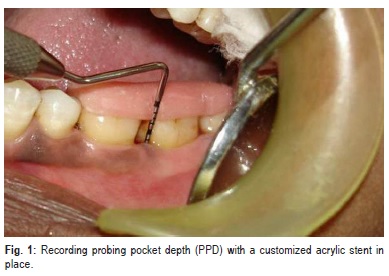
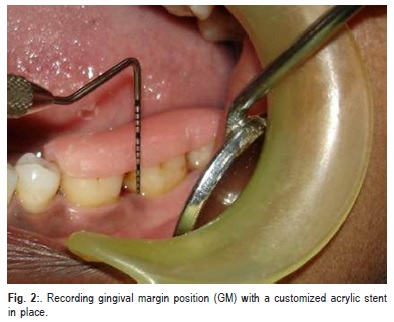
Patients were evaluated clinically and radiographically pre-surgically at baseline and post-surgically at 6 months. In addition to PI and GI, other clinical parameters recorded include probing pocket depth (PPD) (Figure 1), clinical attachment level (CAL) and gingival margin position (GMP) (Figure 2) .All the measurements were standardized using customized acrylic occlusal stents with grooves, which were prepared on the study model of the patients. The recordings were made using a Hu-Friedy PCP UNC 15 probe.
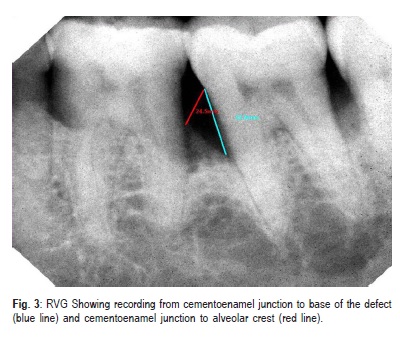
The RadioVisioGraph (RVG), a digital radiography system, was used to obtain radiovisiographic images of each site before surgical procedure and at 6 months postoperatively using the parallel cone technique. For this purpose, a Dexis CCD Sensor (Provision Dental System, Indonesia) was placed inside disposable polythene sleeve and positioned in the mouth. The radiovisiographic images stored in JPEG format were transferred to VixWin PRO software. The cementoenamel junction (CEJ), the base of the defect (BD) and the alveolar crest (AC) were located on the image. Using VixWin PRO Software lines were drawn from CEJ to BD and from CEJ to AC respectively (Figure 3). The distance between the two points of each line were displayed on screen. The depth of the osseous defect was obtained by subtracting the two measurements. The radiographic measurements were obtained for each site by using the above mentioned method for preand postsurgical radiographs. All clinical and radiographic measurements were performed by a single examiner who was blinded to the procedure.
Surgical procedure
All surgical procedures were performed by a single surgeon. Following a pre-surgical rinse with 10 mL 0.2%
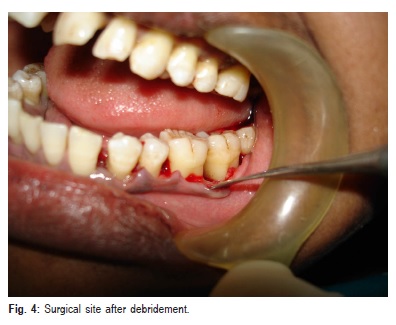
chlorhexidine gluconate solution for 1 min, a conventional surgical approach consisting of periodontal access flap was initiated with intracrevicular (sulcular) incisions on the buccal and lingual aspect of the involved tooth. The incisions were carried as far interproximally as possible to preserve the entire interdental papilla in order to achieve favorable wound closure. The flap was extended to include one tooth mesial and one tooth distal to the tooth associated with the defect. A full thickness mucoperiosteal flap was reflected using the periosteal elevator taking care that the interdental papillary tissue was retained as far as possible. After reflection of the flap and exposure of osseous defect, a thorough surgical debridement of soft and hard tissue was done using the area specific Gracey curettes (Figure 4). After completion of debridement, the surgical site was irrigated copiously with 0.9% normal saline. After isolation of the site, root surface biomodification was done by burnishing the root surfaces with cotton pellets soaked in freshly prepared saturated solution of tetracycline hydrochloride at a concentration of 100 mg/mL for 3-5 min. Root surfaces were then irrigated profusely with normal saline.
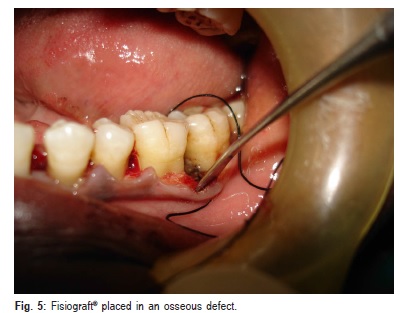
At the experimental site, presuturing was done. Both powder and gel form of Fisiograft ® were taken out from the sterile pack and mixed to the required quantity in the sterile tray which is provided with the powder to obtain a mixture and to make it easier to place in osseous defects. The mixed material was placed into the osseous defect with the scoop end of the cumine scaler (Hu-Friedy) incrementally with light pressure and filled upto the most coronal level of the osseous wall (Figure 5).
The mucoperiosteal flaps were repositioned and secured in place using a 26 mm 3/8 reverse cutting needle and 3-0 non absorbable Mersilk black braided silk surgical suture (Ethicon, Johnson and Johnson Ltd., Somervile, NJ, USA). Interrupted direct loop sutures were placed to obtain primary closure of the interdental space and the area was protected with Coe-pak TM ® periodontal dressing (GC, Inc., Alsip, IL, USA). At the control sites, same surgical procedure was performed but without the placement of graft. Care was taken to maintain asepsis throughout the surgical procedure.
Post-surgical infection control and maintenance oral care
Antibiotic coverage consisting of 500 mg of Amoxicillin three times a day and analgesic consisting of combination of Ibuprofen (325 mg) and Paracetamol (400 mg) were prescribed for five days. Patients were instructed to rinse twice daily with 0.2% chlorhexidine gluconate for 6 weeks. The periodontal dressing and sutures were removed 10 days postsurgery. During this period, the patient was restrained from mechanical oral hygiene procedure and chewing in the treated area. The patients were recalled at one, 3 and 6 months postsurgery at which time oral hygiene instructions were reinforced and full mouth professional prophylaxis in the form of supragingival scaling with ultrasonic instruments was done.
Statistical analysis
Changes in the clinical and radiovisiographic parameters from baseline to 6 months postsurgery in both experimental and control groups were analyzed by Wilcoxon Signed Rank test within a group (intra-group); and inter-group comparisons were made by Mann-Whitney U-test. For all the tests, a pvalue of 0.05 or less was considered for statistical significance.
Results
One out of 8 patients (one experimental and one control site) dropped out of the study for unknown reasons. Hence, the data of this patient were excluded from the study.
Postoperative wound healing was uneventful during the course of study. There were no untoward effects, allergic reactions, infections or patient complaints related to the graft material. The synthetic copolymer used proved to be extremely easy to handle as a result of combination of gel and powder which easily adapts.
PI score reduced by 12.5%, which was not statistically significant (p>0.05). In GI score, a statistically significant reduction of 20.4% was obtained (p<0.01).
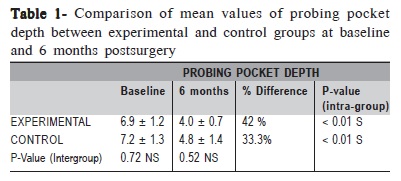
The changes in mean PPD were found to be statistically significant within the groups (p<0.01) (Table 1). However, comparison between groups revealed that the difference in PPD reduction was not statistically significant (p = 0.72) (Table 1).
The changes in mean CAL were found to be statistically significant (p < 0.01) on both intra-group and inter-group comparisons (Table 2).
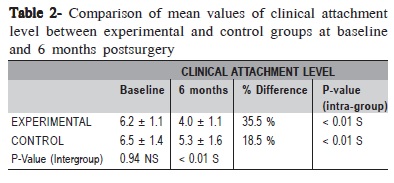
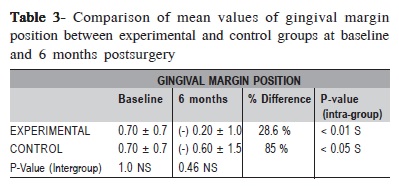
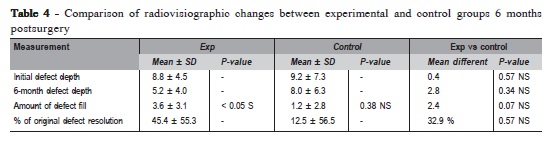
The changes in mean GMP were found to be statistically significant within the groups (p<0.01 in control group, p<0.05 in experimental group) (Table 3). However, comparison between groups revealed that the difference in changes in GMP was not statistically significant (p = 0.46) (Table 3). The initial defect depth in the control group was 9.2 ± 7.3 mm at baseline and at 6 months it was 8.0 ± 6.3 mm which was not statistically significant (p= 0.38). The initial defect depth in the experimental group was 8.8 ± 4.5 mm at baseline and at 6 months it was 5.2 ± 4.0 mm, which was statistically significant (p<0.05). Comparing the amount of defect fill between groups at 6 months postsurgery, it was not statistically significant (p = 0.07). (Table 4). The mean percentage of original defect resolved in the experimental and control groups after 6 months postsurgery was 45.4 ± 55.3 and 12.5 ± 56.5 respectively (p = 0.57).
Discussion
Previously, PLA/PGA polymers have been used to make biodegradable screw pins, rods, plates, suture19. PLA, PGA and their copolymers have been successfully used to fabricate bone substitutes and delivery systems for drugs or osteogenic proteins20. As the polymer degrades in vivo, it serves, not only as a vehicle for drug delivery, but also as a scaffold to support new bone formation. Fisiograft® copolymer has shown the fastest degradation rate of the D-L lactide/glycolide biomaterial in about 50-60 days21. Hollinger22evaluated the healing capacity of experimentally induced osseous defects treated with 50 PLA:50 PGA implants. The hydrolytic degradation of the implants was associated with increasing formation of trabecular bone. Fisiograft® sponge implanted in the post-extraction sites resulted in the formation of matured, mineralized and well structured bone after 6 months of healing. Particles of grafted material could not be identified in any of the biopsied site23. In another study where sponge was grafted in extraction sockets to evaluate the degree of resorption after 3 months, results suggested that Fisiograft® did not interfere with the formation of new bone in the alveolar sockets and that the characteristics of the 3- month newly formed bone seemed to be optimal for dental implants insertion. The biocompatibility, safety and
characteristics of Fisiograft ® indicate that the material is suitable for filling alveolar sockets following extractions in order to prevent volume reduction and collapse of the overlying soft tissue flaps12.
In a study that evaluated ultrastructurally the healing pattern of bone defects in rabbit tibia following the placement of Fisiograft®, it was observed that the material acted in an osteoconductive manner resulting in new bone matrix formation10.
Hassan16 evaluated the efficacy of autogenous bone graft with Fisiograft® on bone healing of buccal dehiscence defects around immediate implants and found that the combination of autogenous bone graft and Fisiograft® showed a slight superiority to autogenous bone graft alone, suggesting that it could be an optimum bone substitute for treatment of dehiscence around immediate dental implant.
In an in vivo study in sockets created in minipigs and filled with Fisiograft® gel and sponge, the results showed that at 30 days Fisiograft® had degraded and the sockets were filled with bone that was not statistically different from normal bone volume fraction, trabecular number and separartion11.
In a histological study13 on sinus lift grafting by Fisiograft® and Bio-Oss®, it was reported that Fisiograft® may be considered both a polymer useful for fastening bone substitutes inside a defect and a material capable of prompting bone regeneration, with or without the use of a bone substitute, having space-former and space-maintainer functions. Fisiograft® has also been show to have potential bone stimulation function, which may be labeled as osteopromotive capability13.
So far, there are only two clinical studies evaluating the effect of PLA/PGA copolymer Fisiograft® in the treatment of deep periodontal intrabony defects in humans. Minenna et al.24 used Fisiograft® sponge in intrabony defects in comparison to OFD and found no significant differences in any of the clinical parameters between the two groups in a 12-month period. Stratul et al.25 in a 6-month study using powder, gel, sponge or powder mixed with gel in intrabony defects compared to OFD found that test group resulted in statistically higher PPD reductions and CAL gains than the control group. Hence, the present study was undertaken to evaluate Fisiograft® efficiency as a regenerative material in periodontal interproximal defects using clinical and radiographic methods. No re-entry was planned at postoperative visit because of ethical considerations as well as associated patient non-acceptance. No complication was seen either indicating that the material is safe to be used clinically.
PI and GI scores decreased from baseline to 6 months, indicating the maintenance of a good oral hygiene by the patient.
In both groups, PPD reduction was found to be statistically significant after 6 months. Although comparison between the groups was not statistically significant, the experimental group showed a higher PPD reduction (42%) as compared to the control group (33.3%).This finding is in accordance with the study by Minenna et al.24 However, Stratul et al.25 reported that changes in relation to PPD reduction showed significant results between the treatments.
In both groups, CAL gain was found to be statistically significant after 6 months. On comparison between the groups at 6 months postsurgery, the difference in CAL gain was 1.3, which was also statistically significant (p < 0.01).The comparison showed higher gain in CAL for the experimental group (35.5%) as compared to control group (18.5%).This finding is in accordance with the study by Stratul et al25. However, according to Minenna et al.24 changes in CAL showed no significant difference between the experimental and control groups at 12 months, but at 6 months statistically significant gain in CAL was seen. (p=0.019).
In both groups, the change in GMP was found to be statistically significant after 6 months. However, on comparison between groups the mean change in GMP at 6 months between the groups was 0.4, which was not statistically significant (p = 0.46). The comparison showed higher change in GMP for the control group (85%) as compared to experimental group (28.6%). This change in GMP occurred due to greater gingival recession in the control group. This finding is in accordance with an earlier study25 and in contrast with another study24.
Radiographic bone measurement following regenerative procedures is a non-invasive, painless alternative to direct bone measurements. Conventional radiography offers simplest and most cost-effective radiographic method but has limited sensitivity and potential geometric distortion. The alternative to intraoral film radiography is RVG. RVG uses an intraoral sensor in place of the radiographic film to produce instant images on a monitor screen. It not only produces instant images on a screen, but also supposedly requires only 20% of radiation needed for conventional radiography26. Additionally, it also has the advantage of control of contrast, ability to enlarge specific areas, potential for computer storage and the subsequent transmission of the images27. Measurement of periodontal intrabony defects from a fixed reference point to the base of the alveolar defect has some value for monitoring osseous changes following regenerative treatment28.
The defect fill was obtained by subtracting postoperative defect depth from the initial defect depth. Comparison between control and experimental groups at 6 months postsurgery revealed that the amount of the defect fill was not statistically significant (p = 0.07). Only one study25 showed a minute or no radiographic defect fill at 6 months after treatment in both control and experimental groups in intrabony defects in humans. The percentage of defect resolved was obtained by subtracting percentage of alveolar crest changes from the percentage of original defect filled. Comparison between control and experimental groups showed that the mean difference in percentage of original defect resolved 6 months postsurgery was 32.9%, which was not statistically significant (p>0.05). The radiographic findings have shown better results in the experimental group wherein percentage of original defect fill was found to be statistically significant compared to the control group. However, the amount of defect fill and percentage of original defect resolved, though not statistically significant, was more than three times higher in the experimental group compared to the control group.
Overall, the findings of this study suggested that placement of Fisiograft® resulted in better healing of osseous defects as assessed clinically and radiographically when compared to OFD alone. However, further long-term studies can better evaluate the regenerative capabilities of this material by histological methods or surgical re-entry. These studies should determine the efficacy of Fisiograft® as well as investigate whether and to what extent Fisiograft® may exert an influence on the healing dynamics following periodontal re-constructive procedures.
References
1. Polson AM. Periodontal regeneration. Current status and directions. Chicago: Quintessence; 1994. p.71,103-4,107. [ Links ]
2. Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976; 47: 256-60.
3. Robert PM, Maudlt J, Frank RM, Vert M. Biocompatibility and resorbability of a resorbable polylactic acid membrane for periodontal guided tissue regeneration. Biomaterials. 1993; 14: 353-8.
4. Robert PM, Frank RM. Periodontal guided tissue regeneration with a new resorbable polylactic acid membrane. J Periodontol. 1994; 65: 414-21.
5. Lundgren D, Nyman S, Mathisesn T, Isaksson S, Klinge B. Guided bone regeneration of cranial defects, using biodegradable barriers: an experimental pilot study in the rabbit. J Craniomaxillofacial Surg. 1992; 20: 257-60.
6. Winet H, Hollinger J.O. Incorporation of polylactide /polyglycolide in a cortical defect: neoosteogenesis in a bone chamber. J Biomed Mater Res. 1993; 27: 667-76.
7. Olson RAJ, Roberts DL, Odon DB. A comparative study of polylactic acid, gelform, and surgical, in healing extraction sites. Oral Surg Oral Med Oral Pathol. 1982; 53: 441-9.
8. Brekke JH, Olson RAJ, Scully JR, Osbon DB. Influence of polylactic acid mesh on the incidence of localized osteitis. Oral Surg Oral Med Oral Pathol. 1983; 56: 240-5.
9. Meadows CL, Gher ME, Quintero G, Lafftery TA. A comparison of polylactic acid granules and decalcified freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1993; 64:103-9.
10. Imbronito AV, Scarano A, Orsini G, Piattelli A, Arana-Chavez VE.Ultrastructure of bone healing in defects grafted with a copolymer of polylactic/polyglycolic acids. J Biomedi Mater Res.. 2005; 74: 215-21.
11. Tschon M, Fini M, Giavaresi G, Rimondini L, Ambrosio L, Giardino R. In vivo preclinical efficacy of a PDLLA/PGA porous copolymer for dental application. J Biomed Mater Res B Appl Biomater. 2009; 88: 349-57.
12. Serino G, Rao W, Iezzi G, Piattelli A. Polylactide and polyglycolide sponge used in human extraction sockets: bone formation following 3 months after its application. Clin Oral Implants Res. 2008; 19: 26-31.
13. Zaffe D, Leghissa GC, Pradelli J, Botticelli AR.Histological study on sinus lift grafting by Fisiograft and Bio-Oss. J Mater Sci Mater Med. 2005; 16: 789-93.
14. Scarano A, Degidi M, Iezzi G, Pecora G, Piattelli M, Orsini G et al. Maxillary sinus augmentation with different biomaterials: a comparative histologic and histomorphometric study in man. Implant Dent. 2006 ; 15: 197-207.
15. Bertoldi C, Zaffe D, Consolo U. Polylactide/polyglycolide copolymer in bone defect healing in humans. Biomaterials. 2008; 29: 1817-23.
16. Hassan KS.Autogenous bone graft combined with polylactic polyglycolic acid polymer for treatment of dehiscence around immediate dental implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108: 19-25.
17. Silness J, Loe H. Periodontol disease is pregnancy and co-relation between oral hygiene and periodontal conditions. Acta Odontol Scand. 1964; 24: 747-59.
18. Loe H, Silness J. Periodontal diseases in pregnancy. Prevalence and severity. Acta Odontol Scand. 1963; 21: 533-51.
19. Agrawal CM, Niederauer GG, Micallef D.M, Athanasiou KA. The use of PLA-PGA polymers in orthopedics. In: Encyclopedic handbook of biomaterials and bioengineering. New York: Marcel Dekkar; 1995. p.2081-115.
20. Sigurdsson TJ, Nygard L, Tatakis DN, Fu E, Turek TJ, Jin L et al. Periodontal repair in dogs: evaluation of rhBMP-2 carriers. Int J Periodontics Restorative Dent. 1996; 16: 524-37.
21. Eppley BL, Reilly M. Degradation characterstics of PLA-PGA bone fixation devices. J Craniofac Surg. 1997; 8: 116-20.
22. Hollinger JO. Preliminary report on osteogenic potential of a biodegradable copolymer of polylactide (PLA) and polyglycolide (PGA). J Biomed Mater Res. 1983; 17: 71-82.
23. Serino G, Biancu S, Lezzi G, Piatelli A. “Ridge preservation following tooth extraction using a polylactide and polyglycolide sponge as space filler: a clinical and histological study in humans”. Clin Oral Implants Res. 2003; 14: 651-8.
24. Minenna L, Herrero F, Sanz M, Trombelli l. Adjunctive effect of a polylactide/ polyglycolide copolymer in the treatment of deep periodontal intra-osseous defects: A randomized clinical trial. J Clin Periodontol. 2005; 32: 456-61.
25. Stratul SI, Rusu D, Sculean A. Evaluation of the polylactide-polyglycolide copolymer fisiograft® in treatment of deep intrabony defects. Int Poster J Dent Oral Med. 2005; 7: 284.
26. Soh G, Loh FC, Chong YH. Radiation dosage of a dental imaging system. Quintessence Int. 1993; 24: 189-91.
27. Wenzel A. Digital radiography and caries diagnosis. Dentomaxillofac Radiol. 1998; 27: 3-11.
28. Machtei E. Outcome variables in the study of periodontal regeneration. Ann Periodontol. 1997; 2: 229-39.
 Correspondence:
Correspondence:
Abhinav Bansal
Department of Periodontics, Peoples College of
Dental Sciences and Research Centre, Bhopal,
Madhya Pradesh, India. - 462037 Phone: +91-9981524099/
Fax: +917554005315
E-mail: abhinav2805@yahoo.com
Received for publication: September 05, 2010
Accepted: February 17, 2011