Services on Demand
Article
Related links
Share
RSBO (Online)
On-line version ISSN 1984-5685
RSBO (Online) vol.8 n.3 Joinville Jul./Sep. 2011
ORIGINAL RESEARCH ARTICLE
A standardized research protocol for platelet-rich plasma (PRP) preparation in rats
Michel Reis MessoraI; Maria José Hitomi NagataII; Flávia Aparecida Chaves FurlanetoIII; Rita Cássia Menegati DornellesIV; Suely Regina Mogami BomfimV; Tatiana Miranda DeliberadorVI; Valdir Gouveia GarciaII; Alvaro Francisco BoscoII
I School of Dentistry, University Center of Lavras – Lavras – Minas Gerais – Brazil
II Department of Surgery and Integrated Clinic, School of Dentistry of Araçatuba, Sao Paulo State University – Araçatuba – Sao Paulo – Brazil
III School of Dentistry, Federal University of Ceara, Campus Sobral – Sobral – Ceara – Brazil
IV Department of Basic Sciences, School of Dentistry of Araçatuba, Sao Paulo State University – Araçatuba – Sao Paulo – Brazil
V Department of Clinic, Surgery and Animal Reproduction, School of Veterinary Medicine of Araçatuba, Sao Paulo State University – Araçatuba – Sao Paulo – Brazil
VI Course and Program of Post-Graduation in Dentistry, Positivo University – Curitiba – Parana – Brazil
ABSTRACT
Introduction: The urgent need for studies using standardized protocols to evaluate the real biological effects of PRP has been emphasized by several authors. Objective: The purpose of this study was to standardize a methodology for autologous Platelet-Rich Plasma (PRP) preparation in rats. Material and methods: Twenty-four, 5 to 6-month-old, male rats, weighing 450 to 500 g were used. After general anesthesia, 3.15 ml of blood was collected from each animal, via cannulation of the jugular vein. A standardized technique of double centrifugation was used to prepare PRP. PRP samples and peripheral blood platelets were then manually counted using a Neubauer chamber. Student’s t-test was used to compare the differences between the number of platelets in peripheral blood and PRP samples (p < 0.05). In addition, PRP and peripheral blood smears were stained to see platelets’ morphology. Results: All surgical procedures were well tolerated by the animals and they were healthy during the entire experimental period. PRP samples showed higher significantly platelet concentrations than peripheral blood samples (2,677,583 and 683,680 respectively). Conclusion: Within the limits of this study, it can be concluded that the method used produced autologous PRP with appropriated platelet quantity and quality, in rats.
Keywords: platelet counts; growth factors; animal models.
Introduction
Reconstruction of facial skeletal hard tissue defects is a field in markedly evolution. The focus of researches on healing in the 1980s was the collection, handling, and transplant of bone-competent cells while in 21st century, the focus has been the use of growth factors capable of stimulating and supporting those cells 4.
Researchers on the field of bucomaxilofacial surgery has continuously searched for improving bone grafting techniques and tried to find ways to obtain the regeneration of the existing defects through a higher bone density and as quickly as possible 25. In 1998, Marx et al. 21 proposed the local use of platelet-rich plasma (PRP) to accelerate autologous bone grafting maturation. According to these authors, autologous bone grafts with PRP healed more quickly and presented a greater bone density than grafts without PRP.
Dentistry study on PRP has been characterized by a peculiar scientific path, firstly initiating on humans 3,18,21,27 then, followed by animal models 1,8,12,32, and now, by in vitro studies 13,16,17,30. These later, mainly, have demonstrated that controversial outcomes of the in vivo studies had possibly occurred due to the use of inappropriate techniques for PRP preparation. Indeed, several fundamental factors must be considered during PRP preparation to assure its quality, and consequently, its biological effect.
PRP acts by accelerating the process of tissue healing through releasing growth factors inside platelet α-granules 21. Therefore, qualitative and/or quantitative alterations in platelets may potentially affect PRP regenerative capacity. Therefore, the choice of the anticoagulant and coagulant, rotation force and number of centrifugations, time elapsed between the sample activation and its clinical use, and the method of blood collection are some factors affecting PRP biological effect 6,7,20.
The necessity of studies on the assessment of PRP biological effects with standardized methodologies was emphasized by Grageda (2004) 10. This author highlighted that additionally to the careful observation of all technical details during PRP preparation, the selection of an adequate animal model is important.
The aim of this study was to develop a standardized protocol for autologous PRP in rats.
Material and methods
Experimental model
Twenty-four male rates (Rattus norvegicus, albino, Wistar), aged between 5 and 6 months, weighing between 450 and 500 g (Sao Paulo State University, Vivarium of the School of Dentistry of Araçatuba) were used. The animals were kept in an environment with 12 hour cycles of light per day and temperature between 22 and 24ºC. The experimental protocol was approved by the Ethical Committee in Animal Experimentation of the Sao Paulo State University, Campus of Araçatuba. During all the experimental time period the animals ate selected solid food and water ad libitum.
PRP preparation
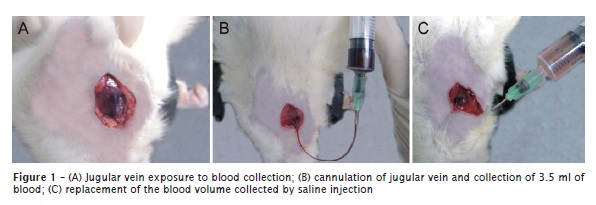
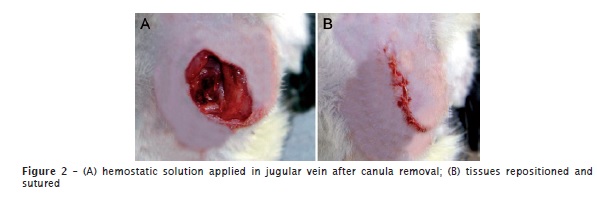
To execute the experimental procedures, the rats were anesthetized through intramuscular injection of xylazine (6 mg/kg) and ketamine (70 mg/kg). The animals were submitted to cannulation via jugular vein, by adapting the technique of Harms and Ojeda (1974) 11. By using a 5 ml disposable syringe containing 0.35 ml of 10% sodium citrate, 3.15 ml of each animal blood was collected (figure 1). The blood was kept in 5 ml silicone vacuum tubes (Vacuum II®, Vitfend Corporation Indústria e Comércio Ltda., Itupeva, SP, Brazil). The same blood amount of each animal was immediately replaced through the injection of sterile saline. The canula placed in the jugular vein was removed and a hemostatic solution was locally applied (figure 2A). The tissues were repositioned and sutured (figure 2B).
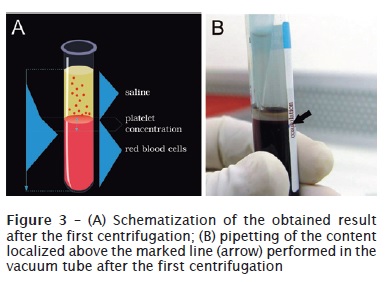
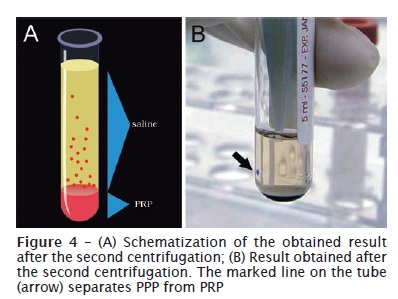
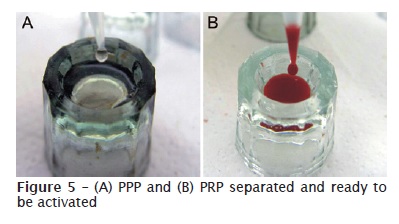
PRP preparation was carried out by adapting the protocol proposed by Sonnleitner et al. (2000) 28, using a refrigerated laboratorial centrifuge (Beckman J-6M Induction Drive Centrifuge, Beckman Instruments Inc., Palo Alto, CA, EUA) and a vertical laminar flow cabinet (Veco®, Veco do Brasil Indústria e Comércio de Equipamentos Ltda., Campinas, SP, Brazil) for manipulating the biological samples. The collected blood was firstly centrifuged at 160 G, for 20 minutes, at environmental temperature (22ºC). Then, a red lower fraction (red cell component) and an upper straw-yellow turbid fraction (serum component) were observed. A point was marked at 1.4 mm below the line dividing the two fractions. All the content above this point was pipetted and transferred to other 5 ml vacuum tube (figure 3), in which a line corresponding to 0.35 ml was drawn from the tube’s bottom 24. The sample was then submitted to a new centrifugation at 400 G, for 15 minutes, resulting in two components: one above the line drawn on the tube (platelet-poor plasma – PPP) and other below the line (PRP) (figure 4) 24. Similar amounts of PRP and PPP (0.35 ml) were pipetted and transferred to different sterile dappen dishes (figure 5). Following, they were activated by 0.05 ml of 10% calcium chloride solution (ScienceLab.com Inc., Houston, TX, EUA) to each 1 ml of PRP or PPP.
Platelet counting
The platelets within animal’s peripheral blood and PRP samples were counted manually in a Neubauer chamber, through binocular optical microscope, at 40X magnification objective lens. For this purpose, each blood sample was separated, diluted and homogenized in Brecher liquid. Moreover, peripheral blood and PRP sample smears were performed and stained with quick panoptic (LB, LaborClin, Pinhais, PR, Brazil).
Statistical analysis
The significance level of the differences between the platelet amount present in peripheral blood and PRP samples was determined through Student’s t test (p < 0.05).
Results
Clinical follow-up
All surgical procedures were well tolerated by the animals and they were healthy during the entire experimental period. Postoperative period was uneventful.
Study on platelet counting
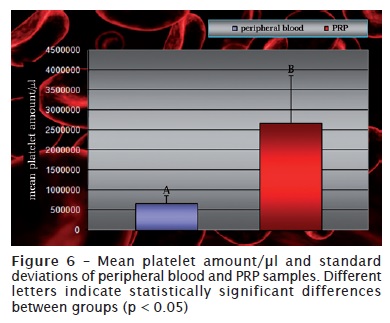
Platelets presented their normal morphology. PRP smears exhibited a greater platelet amount than peripheral blood smears. Mean platelet amount within animal’s peripheral blood was 683,680 ± 186,229 x 103 platelets/μl, while PRP samples presented a mean of 2,677,583 ± 1,201,418 x 103 platelets/μl (figure 6). Therefore, mean PRP platelet amount was about four times greater than that observed within peripheral blood samples.
Discussion and conclusion
The use of inappropriate experimental models and the lack of standardization of a PRP preparation protocol make difficulty the establishment of definitive conclusions on its real biological effect. The aim of this study was to develop a standardized PRP preparation protocol in an experimental model of easy use.
This study was planned taking into account the amount and quality of the platelets within PRP samples. Concerning to the platelet amount, the protocol of double centrifugation used in this study, as recommended by Marx (2001) 19, enabled a mean percentage increase of about 390% in PRP platelet amount in comparison with peripheral blood platelet amount. According to Marx (2004) 20, this platelet concentration within PRP would be considered as therapeutical, and capable of hard and soft tissue regeneration. Animal studies demonstrated that the use of therapeutic PRP samples accelerated bone healing 14,31.
Regarding to platelet amount, PRP samples smears showed that the used PRP preparation protocol did not promote any alteration in platelet morphology. According to Marx et al. (2004) 20, any damage to platelet membrane during PRP preparation will resulting in secretion of growth factors in a non bioactive state, which would lead to unfavorable clinical outcomes. To assure concentrated platelets’ quality, it is necessary to consider the anticoagulant and the velocity used for centrifugation, among other factors. We employed sodium citrate as anticoagulant. Sodium citrate does not alter the platelets’ membrane, allowing that the sample’s anticoagulated state be changed to a coagulated state by adding calcium chloride solution 2,15. Also, platelets’ activation during blood collection procedures with sodium citrate is smaller than in samples collected with EDTA 7. Concerning to centrifugation velocity, the maximum force used in this present study was 400 G. This centrifugation velocity does not lead to early platelets’ activation during PRP preparation, which would happen when greater forces are employed, resulting in growth factors loss within supernatant plasma 6.
At PRP clinical application moment, it is necessary that the anticoagulated sample be changed to a coagulated state. The activator used for coagulating PRP may interfere in growth factor sequence of releasing and, consequently, in the tissue healing process. Our study used only 10% calcium chloride solution for PRP activation. A positive effect of PRP activated by only this solution was also demonstrated by previous studies that evaluated either the healing of tooth extraction sites 2 or osteoblasts proliferation in vitro 9. Additionally, it is important highlighting that PRP activation by only calcium chloride preserves its autologous nature, avoiding the use of bovine thrombin and its risks of coagulopathy development 5,29,15. In studies conducted by our team, PRP activation only by calcium chloride potentializes the healing of critical-size defects at rat calvaria 22,23.
In addition to all caution regarding to the possible factors compromising PRP biological effect, it is important to consider the experimental model used. According to Marx (2004) 20, some studies evaluate homogenous PRP samples and, therefore, the observed biological responses could have been influenced by the occurrence of immunological reactions. In this present study, it was possible to obtain autologous PRP samples, although the rat is a small animal and has a reduced blood volume. Among the advantages presented by this experimental model animal, the low cost and possibility of using in large amount are emphasized 26.
Every study should involve, fundamentally, the production of a high quality PRP for then evaluate the biological effects produced by it 20. Within the limits of this study, it can be concluded that the used method enabled the production of an autologous PRP with appropriate quantity and quality of platelets, in rats.
References
1. Aghaloo TL, Moy PK, Freymiller EG. Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants. 2004 Jan-Feb;19(1):59-65. [ Links ]
2. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of sites for implants. Int J Oral Maxillofac Implants. 1999 Jul-Aug;14(4):529-34.
3. Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002 Aug;37(4):300-6.
4. Carlson ER. Bone grafting the jaws in the 21st century: the use of platelet-rich plasma and bone morphogenetic protein. Alpha Omegan. 2000 Aug-Sep;93(3):26-30.
5. Cmolik BL, Spero JA, Magovern GJ, Clark RE. Redo cardiac surgery: late bleeding complications from topical thrombin-induced factor V deficiency. J Thorac Cardiovasc Surg. 1993 Feb;105(2):222-8.
6. Dugrillon A, Eichler H, Kern S, Klüter H. Autologous concentrated platelet-rich plasma (CPRP) for local application in bone regeneration. Int J Oral Maxillofac Surg. 2002 Dec;31(6):615-9.
7. Efeoglu C, Akcay YD, Erturk S. A modified method for preparing platelet-rich plasma: an experimental study. J Oral Maxillofac Surg. 2004 Nov;62(11):1403-7.
8. Fennis JP, Stoelinga PJ, Jansen JA. Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg. 2004 Jan;33(1):48-55.
9. Ferreira CF, Gomes MCC, Scarso Filho J, Granjeiro JM, Simões CMO, Magini RS. Platelet-rich plasma influence on human osteoblasts growth. Clin Oral Implants Res. 2005 Aug;16(4):456-60.
10. Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent. 2004 Dec;13(4):301-9.
11. Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol. 1974 Mar;36(3):391-2.
12. Jakse N, Tangl S, Gilli R, Berghold A, Lorenzoni M, Eskici A et al. Influence of PRP on autogenous sinus grafts: an experimental study on sheep. Clin Oral Implants Res. 2003 Oct;14(5):578-83.
13. Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg. 2005 Mar;63(3):362-9.
14. Kim SG, Kim WK, Park JC, Kim HJ. A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg. 2002 Sep;60(9):1018-25.
15. Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000 Mar;58(3):297-300.
16. Landesberg R, Burke A, Pinsky D, Katz R, Vo J, Eisig SB et al. Activation of platelet-rich plasma using thrombin receptor agonist peptide. J Oral Maxillofac Surg. 2005 Apr;63(4):529-35.
17. Leitner GC, Gruber R, Neumüller J, Wagner A, Kloimstein P, Höcker P et al. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. Vox Sang. 2006 Aug;91(2):135-9.
18. Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet-rich plasma, bovine porous bone mineral, and guided tissue regeneration versus platelet-rich plasma and bovine porous bone mineral in the treatment of intrabony defects: a reentry study. J Periodontol. 2002 Feb;73(2):198-205.
19. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-8.
20. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004 Apr;62(4):489-96.
21. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol. 1998 Jun;85(6):638-46.
22. Messora MR, Nagata MJ, Mariano RC, Dornelles RC, Bomfim SR, Fucini SE et al. Bone healing in critical-size defects treated with platelet-rich plasma: a histologic and histometric study in rat calvaria. J Periodontol Res. 2008 Apr;43(2):217-23.
23. Messora MR, Nagata MJ, Dornelles RC, Bomfim SR, Furlaneto FA, Melo LG et al. Bone healing in critical-size defects treated with platelet-rich plasma activated by two different methods: a histologic and histometric study in rat calvaria. J Periodontal Res. 2008 Dec;43(6):723-9.
24. Messora MR, Nagata MJ, Furlaneto FAC, Deliberador TM, Melo LGN, Garcia VG et al. Análise da eficiência do protocolo de dupla centrifugação para o preparo do plasma rico em plaquetas (PRP): estudo experimental em coelhos. RSBO. 2009 Sep;6(3):291-6.
25. Raghoebar GM, Schortinghuis J, Liem RS, Ruben JL, van der Wal JE, Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005 Jun;16(3):349-56.
26. Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop. 1986 Apr;205:299-308.
27. Shanaman R, Filstein MR, Danesh-Meyer MJ. Localized ridge augmentation using GBR and platelet-rich plasma: case reports. Int J Periodontics Restorative Dent. 2001 Aug;21(4):345-55.
28. Sonnleitner D, Huemer P, Sullivan DY. A simplified technique for producing platelet-rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note. Int J Oral Maxillofac Implants. 2000 Nov-Dec;15(6):879-82.
29. Spero JA. Bovine thrombin-induced inhibitor of factor V and bleeding risk in postoperative neurosurgical patients. J Neurosurg. 1993 May;78(5):817-20.
30. Tsay RC, Vo J, Burke A, Eisig SB, Lu HH, Landesberg R. Differential growth factor retention by platelet-rich plasma composites. J Oral Maxillofac Surg. 2005 Apr;63(4):521-8.
31. Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004 Apr;34(4):665-71.
32. Zechner W, Tangl S, Tepper G, Fürst G, Bernhart T, Haas R et al. Influence of platelet-rich plasma on osseous healing of dental implants: a histologic and histomorphometric study in minipigs. Int J Oral Maxillofac Implants. 2003 Jan-Feb;18(1):15-22.
 Correspondence:
Correspondence:
Eduardo Pizzatto
Mestrado em Odontologia – Universidade Positivo
Rua Professor Pedro Viriato Parigot de Souza, n. 5.300 – Campo Comprido
CEP 81280-330 – Curitiba – PR – Brasil
E-mail: epizzatto@up.com.br
Received for publication: December 16, 2010
Accepted for publication: February 12, 2011