Services on Demand
Article
Related links
Share
RSBO (Online)
On-line version ISSN 1984-5685
RSBO (Online) vol.9 n.1 Joinville Jan./Mar. 2012
ORIGINAL RESEARCH ARTICLE
Evaluation of cleaning efficacy of a nickel-titanium rotary system, with or without 17% EDTA passive ultrasonic activation: a scanning electron microscopic study
Antônio Henrique Braitt I; Rodrigo Sanches Cunha II; Alexandre Sigrist de Martin I; Carlos Eduardo da Silveira Bueno I
I São Leopoldo Mandic School of Dentistry – Campinas – SP – Brazil.
II University of Manitoba – MB – Canada.
ABSTRACT
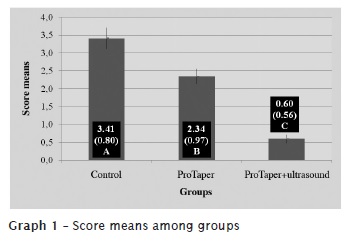
Introduction: The goal of endodontic instrumentation is to promote root canal cleaning and shaping to prepare it for the subsequent three-dimensional filling. Objective: The aim of this study was to evaluate, ex vivo, root canal cleaning ability executed by nickel-titanium rotary system instrumentation and this same system plus ultrasound passive activation of 17% EDTA, through SEM. Material and methods: Seventy upper second single-rooted human bicuspids were used. All teeth presented a single root canal, flattened towards buccal-palatal direction. The teeth were randomly separated into 3 groups. Group 1 (n = 30), had the canals instrumented by using the original operative sequence of ProTaper Universal System, up to instrument #F3. In this group, 5 ml of 5.25% sodium hypochlorite was employed as irrigant, every each instrument change. After group 1 instrumentation, root canals were irrigated with 5 ml of 17% EDTA, which was kept inside the canal for 3 minutes. Next, a final irrigation with 5 ml of 5.25% NaOCl was performed to remove the smear layer in suspension. Group 2 (n = 30) had the canals instrumented by the same system and up to the same instrument size. It was used 5.0 ml of 5.25% NaOCl as irrigant substance every each instrument change. In group 2, however, 17% EDTA (5 ml) was applied through ultrasonic passive activation for 1 minute, and then leaving the substance for 2 minutes within root canal.A final irrigation and with 5.25% NaOCl was also performed. Group 3 (n = 10) was the control group, where the canals were not instrumented and irrigation was executed with saline solution. After that, the teeth were cut into their long axis, metalized and taken for SEM analysis, at x2000 magnification. Each tooth's cervical, middle and apical thirds were observed. The cleaning quality of root canal's walls was observed by the images analyzed by three examiners. Results: Data were statistically analysed by analysis of variance and Tukey test, with a significance level of 5%. The control group showed an average score of the presence of smear layer of 3.41; group 1 (ProTaper) of 2.34; and the group 2 (ProTaper+Ultrassound) of 0.60. Conclusion: None of the studied preparation techniques promoted the total cleaning of the root canal walls. The addition of the ultrasound passive activation, after rotary instrumentation, promoted an increase of the smear layer removal, improving the cleaning of root canal wall. The apical third obtained the smallest cleaning rate, regardless of the technique employed.
Keywords: irrigation; rotary instrumentation; ultrasound.
Introduction
Endodontic treatment goal is the cleaning, shaping and tridimensional filling of root canal systems. Root canal cleaning and shaping is performed by both the kinematic action of intracanal instruments and the irrigants. It is an important phase of the endodontic treatment because it aims the root canal disinfection, contributing for endodontic treatment success 4,10,11.
While the cleaning and shaping procedures involve the emptying and preparing of root canal lumen to receive the posterior obturation regardless of the pulp's clinical status, the sanitation process is obtained by the action of endodontic instruments together with the irrigant. This adjuvant substance should not only involve the cutting dentin, but also remove the smear layer within root canal's wall, fighting the existing microorganisms. Additionally, the irrigant should have a superficial tension enabling it to penetrate within the dentinal tubules, lateral canals, secondary canals, inter-canals, recurrent canals, and apical delta, promoting the cleaning of all root canal system.
The knowledge on biology and mechanics associated with Endodontics enable the execution of a new endodontic science based on applied biology, consequently resulting in a better clinical developing for the dentist, greater comfort for the patient and treatment with cost-benefit which assumes a relevant role in currently daily practice. These current technologic and biologic advancements no longer allow the segregation of the basic knowledge from the clinical practice because one must never dissociate such knowledge from the elements that govern the endodontic treatment today 18.
Ultrasonic devices appear in the endodontic armamentarium and evolve in such way that they are indispensable adjuvants in root canal system cleaning 1,12.
Although effective, rotary instrumentation demands a copious irrigation together with the instruments' action, failing to leave organic and inorganic material adhered to root canal wall (smear layer), material in putrefaction and microorganisms, which impedes root canal sanitation and makes unviable a complete obliteration during endodontic obturation. Studies demonstrated that the smear layer removal may be optimized with the use of ultrasonic cavitation 13.
Therefore, since the last years have been prolific regarding to the advancement of root canal system cleaning and shaping, it is important to study the effect of the association of technologies such as rotary instrumentation with nickel-titanium instruments and ultrasonic cavitation on the removal of the smear layer formed during root canal instrumentation.
Material and methods
This study project was approved by the Ethical Committee in Research of the São Leopoldo Mandic School of Dentistry and Research Center in February 18, 2009, under protocol 2008/0259.
Seventy single-rooted human bicuspids with only one root canal flattened at buccal-palatal direction, donated by the Tooth Bank of São Leopoldo Mandic Dental Research Center, were used. The teeth were radiographed at mesial-distal and buccal-lingual direction and kept in 2.5% sodium hypochlorite, for one hour for cleaning and disinfection. Following, the teeth were put under running water for one hour to remove sodium hypochlorite excess and kept in flasks containing 0.1% thymol solution.
At the moment of their use, the teeth were washed under running water and kept in flasks with saline, dried with air jet and gauze, cut into transversal direction at the level of their anatomic neck, keeping the roots with 15 mm to standardize the specimens' size. Next, the teeth were randomly divided into three groups, according to the chart below (figure 1).
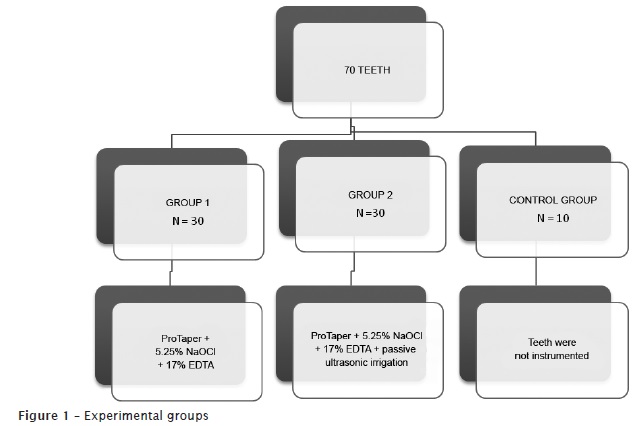
Teeth of group 1 (n = 30) were mounted into a counter for endodontic training and had their root canals instrumented with the original operative sequence of the ProTaper Universal System (Dentsply/Maillefer, Switzerland) up to the instrument size #F3. The instruments were mounted in an Endo-Mate 2 motor at a velocity of 300 rpm and torque of 3 Ncm². As irrigant solution, it was employed 5.0 ml of 5.25% sodium hypochlorite (Viapharma Farmácia de Manipulação Ltda., Brazil). Irrigation/aspiration were executed through a 5 ml plastic disposable syringe + 0.55X20 needle and the suction tip of the dental unit (KaVo do Brasil S.A.) at every instrument change (SX, S1, S2, F1, F2 and F3).
After instrumentation, irrigation/aspiration was performed with 17% EDTA (Viapharma Farmácia de Manipulação Ltda., Brazil) in aqueous vehicle, buffered with sodium hydroxide pH 7.0. This substance was kept within root canal for 3 minutes. Next, a final irrigation/aspiration with 5 ml of 5.25% sodium hypochlorite was executed to remove the suspended smear layer.
In group 2 (n = 30), we performed the same aforementioned procedures with the exception that 17% EDTA in aqueous vehicle (5 ml) buffered with sodium hydroxide pH 7.0 was inserted with a slow, constant, and continuous irrigation/aspiration for 1 minute, and then leaving the substance for 2 minutes within root canal. The passive irrigation was activated by ST 21B file mounted into an ultrasonic device (OE5, Enad Osada, Japan) at amplitude #3 from the wave dial, penetrating 12 mm into root canal, in a way to avoid root canal wall. Following, a final irrigation with 5 ml of 5.25% sodium hypochlorite was executed to remove the smear layer in suspension.
The teeth from group 3 (control group) did not have their canals instrumented. They were only irrigated with 5.0 ml of saline.
Vertical slots were performed at the distal and mesial surfaces through steel disks mounted in slow-speed handpiece. With the aid of a LeCron spatula, the teeth were vertically cleaved at mesial-distal direction. Therefore, two root hemi-sections were obtained, exposing the prepared root canal lumen.
The root hemisection that presented the best possibility of visualization was selected and posteriorly stored into an incubator at 45ºC for 12 hours to dehydrate the specimens. This would favor the process of metallization needed to scanning electron microscopy (SEM) visualization.
The metallization of the specimens were executed through the use of Denton Vacuum Desk II metalizer. A thin gold layer was applied for SEM analysis using the conventional method (high vacuum). For this purpose, the samples were fixed with the aid of a specific conductive double-sided carbon tape into a stub and taken to the metalizer device.
The metalized samples were seen at SEM (JEOL JSM5600lV). The initial image was first seen at x50 magnification and then at x 2,000 magnification, observing the root canal third (cervical, medium, or apical) to be analysed. The most representative images were selected, then analysed in a computer screen, and stored in digital format (.bmp).
The images were analysed by three examiners who received a CD containing the digitized images, numbered and disposed in a Power Point software presentation. The information regarding to which experimental group and which third the images corresponded was not provided. Also in the CD, a second Power Point presentation was available containing five SEM digital images to be used as example of the scores to be attributed to the study's images. The score images were disposed in descending order of cleaning, from 0 to 4, according to the criteria adapted from those of Hülsmann et al. 9:
0 – a completely clean surface, with all dentinal tubules completely cleaned or rare presence of smear layer;
1 – visible dentinal tubules, but with presence of smear layer dispersed by the dentinal wall;
2 – surface covered by a thin smear layer with most of dentinal tubules exposed;
3 – surface covered with a thick smear layer, with rare exposed dentinal tubules;
4 – surface totally covered with smear layer, without the possibility of dentinal tubules visualization.
These criteria allowed that the examiners could score the digital images regarding to the quality evaluation of the root canal wall cleaning.
A mean value of the three scores of each image was calculated. Data was statistically analysed by analysis of variance for repeated measures and calculated through a mixed model for the variables group, third, and interaction. The comparison of the means was executed by Tukey test and the level of significance was set at 5%. Statistics was performed by SAS system (SAS Institute Inc. The SAS system, release 9.2 – TS Level 2MO. SAS Institute Inc., Cary:NC.2008).
Results
The results obtained by the qualitative evaluation allowed a trustable comparison among groups.
By interpreting the data provided by Tukey test, at level of significance of 5%, we found statistical significant differences in the presence of smear layer between the studied technologies. The highest presence of smear layer was found in control group, followed by ProTaper alone and ProTaper + ultrasound (graphs 1 and 2).
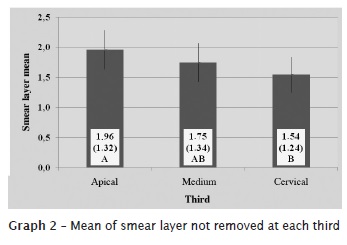
The obtained results are seen in figures 2, 3, and 4.

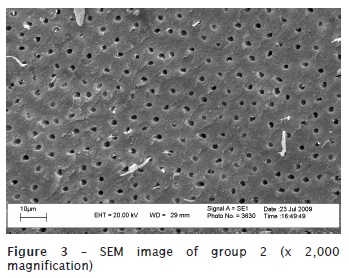
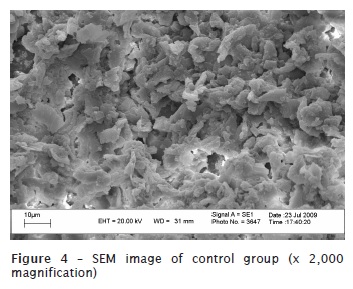
Discussion
Nickel-titanium instruments driven by electrical motors have facilitated endodontic treatment by reducing the operator's fatigue and the patient's stress 11,14,15,16,17.
Despite of the efficiency, rotary instrumentation of root canals demands a copious irrigation together with the instruments use because although it promotes a greater amount of removed dentin due to its more accentuated taper, it does not promote according to some studies an effective cleaning of root canal's wall, particularly in apical third, curvatures 9, and oval-shaped canals 15,16, even presenting a centralized and symmetric preparation, avoiding perforations and communication with the periodontium 7 and maintaining a better root canal shaping 4.
Aiming to improve root canal sanitation and to aid the smear layer removal during root canal cleaning and shaping, we performed rotary instrumentation together with passive ultrasonic activation, resulting in a better root canal cleaning because it removes the residual dentin and debris layer which would be retained within the wall during instrumentation 1,6.
In the comparison among groups, we aimed to standardize the several study's variables: type of tooth; shaping diameter; rotary instrumentation system; type, amount, and concentration of the irrigant; ultrasound type, device, and tip; amount and concentration of the final irrigant; specimen's section; specimens' preparation for SEM evaluation; SEM magnification for specimens' analysis.
Concerning to the specimens' preparation for SEM evaluation, we realized the need of dehydrating the samples. Due to their rigidity, dehydration is executed through air dry in an incubator at 45ºC up to the specimen's critical point, to avoid the appearance of undesirable artefacts, such as the structure's cleavages or cracks. Following, the teeth were submitted to gold metallization in Denton Vacuum Desk II metalizer. In this study an initial x50 magnification for SEM analysis for a general visualization of the root was used and then we chose the root's third to be studied. Next, a x2,000 magnification was chosen, aiming to obtain a better visualization of dentinal tubules and better assessment of the smear layer presence
Conclusion
Within this study's conditions, it can be concluded that none of the studied preparation techniques promoted a total cleaning of root canals'walls. Passive ultrasonic activation after rotary instrumentation resulted in an increase of smear layer removal, improving root canal wall cleaning. SEM analysis showed that the apical third obtained the smallest cleaning rate in comparison with the other thirds, regardless of the preparation technique employed.
References
1. Al-Jadaa A, Paqué F, Attin T, Zehnder M. Necrotic pulp tissue dissolution by passive ultrasonic irrigation in simulated accessory canals: impact of canal location and angulation. Int Endod J. 2009;42:59-65. [ Links ]
2. Braitt AH. Saneamento dos canais radiculares. RGO.1980;28:200-2.
3. Braitt AH. Considerações sobre o uso de aparelhos ultra-sônicos em Endodontia. Rev Odonto. 1992;2:242-6.
4. Calberson FLG, Derose CAJG, Hommez G MG, De Moor RJG. Shaping ability of ProTaper nickel-titanium files in simulated resin root canals. Int Endod J. 2004;37:613-23.
5. Cameron JA. Factors affecting the clinical efficiency of ultrasonic endodontics: a scanning electron microscopy study. Int Endod J. 1995;28:47-53.
6. Esberard RM, Leonardo MR, Leal JM, Simões Filho AP, Bonetti Filho I. Ultrassom em Endodontia. RGO 1987;36:297-300.
7. Fabra-Campos H, Rodriguez-Vallejo J. Digitization, analysis and processing of dental images during root canal preparation with Quantec series 2000 instruments. Int Endod J. 2001;34:29-39.
8. Garcia PFB, Lopes HP, Lithgow CV. Avaliação do deslocamento do preparo do canal após emprego de instrumentos endodônticos de aço inoxidável e de níquel-titânio da Série 29. JBE. 2000;1:27-31.
9. Hülsmann M, Rümmelin C, Schäfers F. Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endod. 1997;23:301-6..
10. Loiola LE, Tanomaru JML, Morgental RD, Tanomaru Filho M. Influência da agulha irrigadora e da dilatação do canal radicular na eficiência da irrigação endodôntica. RSBO. 2011;8(2):138-44.
11. Loizides AL, Kakavetsos VD, Tzanetakis GN, Kontakiotis EG, Eliades G. A comparative study of the effects of two nickel-titanium preparation techniques on root canal geometry assessed by microcomputed tomography. J Endod. 2007;33:1455-9.
12. Lumley PJ, Walmsley AD, Ealton RE, Rippin JW. Cleaning of oval canals using ultrasonic or sonic instrumentation. J Endod. 1993;19:453-7.
13. Peters OA, Peters CI, Schönenberger K, Barbakow F. ProTaper rotary root canal preparation: assessment of torque and force in relation to canal anatomy. Int Endod J. 2003;47:93-9.
14. Prati C, Foschi F, Nucci C, Montebugnoli L, Marchionni S. Appearance of the canal walls after preparation with NiTi Rotary instruments: a comparative SEM investigation. Clin Oral Investigations. 2004;8:102-10.
15. Rüttermann S, Virtej A, Raab WHM. Preparation of the coronal and middle third of oval root canals with a Rotary or an oscillating system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:852-6.
16. Schäfer E, Lohmann D. Efficiency of rotary nickel-titanium FlexMaster instruments compared with stainless steel hand K-Flexofile. Part 2. Cleaning effectiveness and instrumentation results in severely curved root canals of extracted teeth. Int Endod J. 2002;35:514-21.
17. Schäfer E, Schlingemann R. Efficiency of rotary nickel-titanium K3 instruments compared with stainless steel hand K-Flexofile. Part 2. Cleaning effectiveness and shaping ability in severely curved root canals of extracted teeth. Int Endod J. 2003;36:208-17.
18. Yamaguchi M, Yooshida K, Suzuki R, Nakamura H. Root canal irrigation with acid solution. J Endod. 1996;22:27-9.
 Correspondence:
Correspondence:
Antônio Henrique Braitt
Avenida Aziz Maron, n.º 1.117, sala 703
CEP 45605-415 – Itabuna – BA – Brasil
E-mail: henrique_braitt@hotmail.com
Received for publication: April 1, 2011
Accepted for publication: July 7, 2011