Services on Demand
Article
Related links
Share
RSBO (Online)
On-line version ISSN 1984-5685
RSBO (Online) vol.9 n.3 Joinville Jul./Sep. 2012
Original Research Article
Bond strength of dentin submitted to bleaching and restored with different materials
Nelize Marcelino Pessarelloo I; Yara T. Correa Silva-Sousa I; Fuad Jacob Abi Rached-Junior I; Aline Evangelista Souza-Gabriel I
ABSTRACT
Introduction: The use of adhesive composite resin with fluoride and with greater fluidity can be favorable to the restoration of the palatal/lingual face of teeth submitted to internal bleaching. Objective: This study evaluated the bond strength of adhesive systems and composite resins to bleached dentin. Material and methods:Forty maxillary canines were sectioned to obtain 40 blocks (5 x 5 mm) of intracoronary dentin. The fragments were included and bleached with 37% carbamide peroxide. After 7 days, the specimens were divided into two groups according to the adhesive system: with (Optibond Solo Plus) and without (Single Bond) fluoride and subdivided into 2 subgroups (n = 10) according to the composite resin: microhybrid (Z250) and flowable (Z350). The restoration was carried out through a bipartite matrix. After 24 hours, the specimens were subjected to shear bond strength test. The data (MPa) were analyzed by ANOVA and Tukey test (a = 0.05). Results: The best results (p < 0.05) were obtained for fluoridated adhesive (7.44 ± 2.35) compared with that without fluoride (5.36 ± 2.01); flowable resin (7.76 ± 2.23) performed better than microhybrid resin (5.03 ± 1.72). When the two variables were associated, the highest results were obtained for the specimens restored with fluoridated adhesive and flowable resin (9.04 ± 1.92). Lower results were observed for non-fluoridated adhesive + microhybrid resin – control (4.24 ± 1.59), without statistically significant differences when compared with the fluoridated adhesive + microhybrid resin (5.83 ± 1.52). Conclusion: The combination with fluoridated adhesive and flowable resin increases the shear bond strength of bleached dentin.
Keywords: aesthetics; bleaching; adhesives; composite resins.
Introduction
Tooth bleaching is a conservative option for the treatment of dark teeth when compared to veneers and indirect crowns 10. The process occurs due to the liberation of oxygen (free radical) by the bleaching agent diffusing through the dentinal tubules and breaking the macromolecules (stained) into increasingly smaller chains (lighter), which are totally or partially eliminated from the tooth structure 10,11.
The bleaching agents most used in endodontically treated teeth have been hydrogen peroxide, carbamide peroxide and sodium perborate, alone or in associations 8,22. Although a high concentration agent is efficient for bleaching the tooth structure, its use has been associated with undesirable complications in hard tooth tissues, including alterations in the permeability of the dentin 5 and in the adhesive capacity of the restorative materials 5,9,15,21.
High concentration agents do not have bleaching power on the restorative materials 1. Frequently, after bleaching, there is the need of changing the previous restorations with the use of adhesive aesthetic restorative procedures 23. Notwithstanding, studies have reported effects of these bleaching agents on the mechanical and morphological characteristics of the adhesive interfaces of bleached teeth 1,2,9,18,17,23. The reduction of the restorative materials bond strength to dentin probably occurs because of the remnants of the bleaching gel inside the dentinal tubules and within the collagen matrix 9,16,24. Moreover, the oxygen releasing may inhibit the light-curing of the composite resins 2,17,20.
The literature has still evidenced changes in the dentinal microhardness after tooth bleaching 3,5,14. There are studies demonstrating the reestablishment of the dentinal microhardness by the application of fluoride on the bleached dentin 3,6,7. Sodium fluoride is the agent mostly employed in this procedure, found as aqueous solutions, gels, varnishes, prophylactic paste and devices of slow fluoride releasing 4. The adhesive containing fluoride in its composition appeared with the aim of inhibiting secondary caries lesions; however it is speculated that these adhesive may also reestablish the materials bond strength to the bleached dentin 12.
The composite resins has undergone modifications in their physical and mechanical properties, in an attempt to minimize the efforts generated on the bonding interface 25. The flowable composite resins show smaller filler concentration, good flowing and low elasticity modulus which theoretically would support and dissipate better the stress generated by thermal and masticatory tensions, favoring the adaptation of the interface 2,25.
Thus it is important to define the best adhesive system as well as the most appropriate material to restore teeth bleached by high concentration agents. The aim of this study was to evaluate in vitro the bond strength of the dentin submitted to bleaching with high concentration agent and restored with different materials, through shear bond strength test and the analysis of the failure type.
Material and methods
Sound maxillary human canines kept in 0.1% thymol solution at 9°C were washed under tap water for 24 hours to eliminate the thymol remnants and examined macroscopically with the aid of a stereoscopic magnifying glass (Leica Microsystems, Wetzlar, Germany) at x 20 magnification. Exclusion criteria comprised the presence of either fracture lines or fissures in tooth crown. Therefore, 40 teeth were selected.
The teeth were embedded into dental utility wax (Polidental, Cotia, SP, Brazil), and cross-sectioned at the enamel-cement junction to separate the crowns from the roots. Following, the tooth crowns were sectioned longitudinally at mesial-distal direction, with the aid of a double-faced diamond disc (KG Sorensen, Barueri, SP, Brazil) coupled into a low-speed straight handpiece (Dabi Atlante, Ribeirão Preto, SP, Brazil). Each crown hemi-section was again cut with the aid of the diamond disc at the incisal, mesial, distal and cervical surfaces to obtain two blocks (5 mm x 5 mm), resulting in 25 mm². Therefore, 40 specimens were obtained.
The specimens were embedded into self-cured acrylic resin (JET Clássico, São Paulo, SP, Brazil), with the aid of PVC rings (1.5 cm of inner diameter and 1.5 cm of height), previously covered with Vaseline, so that the intracoronary dentinal surface remained turned to the external environment.
After the acrylic resin curing, the rings were removed and the surface of the specimens were flattened with the aid of 280- and 400-grit silicon carbide sandpapers (Norton, Lorena, SP, Brazil), under copious irrigation. Next, the dentinal surface underwent 60 standardized cycles of sanding through 1200-grit sandpaper, to obtain the smear layer, to simulate the clinical situation. The specimens were washed by 10 ml of 1% sodium hypochlorite for 10 minutes, aiming to simulate the irrigation during the biomechanical preparation of the root canals.
The specimens were than bleached with 37% carbamide peroxide (Whiteness Super, FGM, Joinville, SC, Brazil) 24. Two bleaching procedures were performed at a 7 day interval between them. At each bleaching procedure, the agent was applied 3 times with an interval of 10 minutes among them. Between each bleaching procedure, the specimens were restored with a provisional restoration 15. After bleaching, the specimens were kept under relative humidity at 37°C for 10 days 16.
Following, the specimens were randomly divided according to the adhesive system to be used (n = 20): Adhesive containing fluoride – Optibond Solo Plus (Kerr, MN, USA) – and adhesive without fluoride – Adper Single Bond 2 (3M, ESPE St. Paul, MN, USA). The specimens were etched with 37% phosphoric acid (3M, ESPE St. Paul, MN, EUA) for 15 seconds, washed for the same time amount and dried with absorbent paper. Both adhesive systems were then applied and light-cured according to each manufacturers instruction.
The dentinal surfaces were subdivided according to the composite resin to be used for the restoration: microhybrid composite resin – Filtek Z250 (3M ESPE) – and flowable resin – Filtek Z350 Flow (3M ESPE). The specimens were restored with the aid of a bipartite Teflon matrix (3 mm of inner diameter., 4 mm of height) stabilized with the aid of silicone impression material (Perfil Denso, Vigodent, Bonsucesso, RJ, Brazil), to obtain composite resin cylinders with the aforementioned measurements. The composite resin was inserted in three increments with the aid of a insertion spatula (Duflex, Rio de Janeiro, RJ, Brazil), light-cured for 20 seconds per each increment, leaving the tip of the optical fiber of the device at 10 cm above the resin surface with the aid of customized device. Next, the silicon barrier was removed with the aid of scalpel blade, the bipartite matrix opened and the specimens kept under relative humidity at 37°C for 24 hour, to be submitted to the shear bond strength test. Figure 1 shows the construction of the specimens for the shear bond strength test.
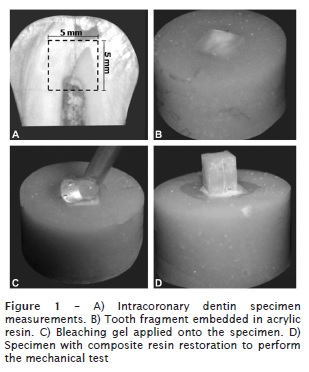
Elapsed the 24 hours, the specimens were placed in a universal testing machine (Instron 4444, Instron Corporation, Canton-Massachusetts, USA), with load of 2 kN, fixed in a stainless steel device, enabling the force incidence at 90°, avoiding the contact with the acrylic resin base of the specimen. The application of the shear bond strength was performed through a rectangular stainless steel tip, at constant speed of 0.5 mm/min up to the dislocation of the restoration.
Data were obtained in kN and transformed into MPa. The strength necessary for the displacement of the restorative material (F), in kilonewtons (kN), was transformed in stress (s) expressed in megapascal (MPa), by dividing the strength force by the adhesion area of the restorative material (A) in mm2. Thus, the formula employed to relate these magnitudes was: s = F / A.
The failures were analyzed through stereoscopic magnifying glass (x40 magnification) (Leica Microsystems, Wetzlar, Germany) and were classified as adhesive (dentinal surface covered by a thin layer of the adhesive material); material cohesive (dentinal surface covered by composite resin); substrate cohesive (failure in dentin); mixed (the combination between adhesive and cohesive types).
Statistical analysis
Data were submitted to preliminary statistical tests, aiming to verify the normality of the sample distribution. As the tested sample was normal and homogenous, it was submitted to statistical parametric two-way ANOVA and Tukey tests, considering the adhesive system and composite resin as independent factors. The level of significance was set at 5% (a= 0.05), with the aid of GraphPad Instat software (GraphPad Software; San Diego, CA, USA).
Results
The statistical analysis revealed a statistically significant difference for the factors adhesive system and composite resin, as well as their interaction (p < 0.05).
The best results (MPa) were obtained for the fluoridated adhesive (7.44 ± 2.35), in comparison with the non-fluoridated adhesive (5.36 ± 2.01), and for the flowable (7.76 ± 2.23), in comparison with microhybrid resin (5.03 ± 1.72) (p < 0.05).
When the two variables were associated, it was verified that the fluoridated adhesive and flowable resin group showed the highest bond strength means with statistically significant differences among the other groups (p < 0.05). The group restored with non-fluoridated adhesive and microhybrid resin (control) presented the smallest means with statistical similarity (p > 0.05) with the fluoride adhesive + microhybrid resin (table I).
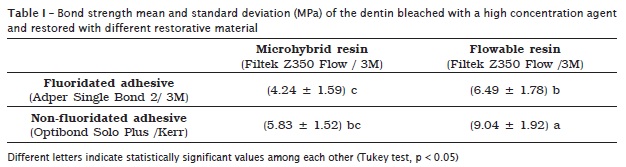
The analysis of the failure type occurring after the shear bond strength test revealed a greater percentage of cohesive failures for the group restored with fluoridated adhesive and flowable resin. The other groups showed adhesive failures predominantly (table II).

Discussion
Internal tooth bleaching has been employed successfully in the treatment of non vital darkened teeth 8,16. Frequently after treatment, there is the need for the change of the previous restorations, through adhesive aesthetic restorative procedures 14,16-18,24. However, the chemical reactions occurring during the tooth bleaching process may alter the tooth structure negatively interfering in the bonding of the restorative systems to tooth substrate 13,22.
In this present study, it was aimed to evaluate the bond strength of the dentin submitted to bleaching with high concentration gel and restored with different materials. The fluoridated adhesive employed in an attempt of reestablishing the bond strength after bleaching and the flowable resin as an alternative to the conventional resin (microhybrid), simulating the restoration on the palatal and lingual surface of the anterior teeth.
In this present study, the strength was determined through shear bond strength test, in which the restoration is disrupted by a force applied parallely to the interface. Shear bond strength test has been largely employed in laboratorial studies 6,13,17-20,24 mainly because of the possibility to standardize the specimens to enable a better distribution of the stress on the adhesive interface.
The bleaching and restorative protocol followed the manufacturers instructions for each material. Between each bleaching procedure, the specimens were restored with provisional cement and kept at 37°C in artificial saliva, to simulate the clinical situations 24.
In this study, the best results were obtained by the fluoridated adhesive in comparison with the non-fluoridated adhesive, and by the flowable resin in comparison with microhybrid resin. The application of fluoride in the hydroxyapatite molecule forms fluoridated apatite, which is a less soluble molecule 13. This is because the occurrence of a strong electrostatic attraction between calcium and fluoride, resulting in more crystalline and stable apatite with largest crystals 4. Moreover, there is the formation of a layer rich in calcium fluoride which is dissolved during the process, enabling that the fluoride diffuses and is incorporated into the dentin 3,4, contributing in this present study to explain the highest bond strength values of the specimens receiving fluoride adhesive.
The flowable composite resin because of its easier penetration in the angles and irregularities of the dentin promoted a better adhesive interface and consequently greater bond strength 25. The low flowing of microhybrid resins may difficult the adaptation or accommodation to the cavity walls 2, generating microbubbles in the bleached tooth/restoration interface, decreasing the bond strength, especially in a more critical substrate as that submitted to the action of high concentration agents 25.
Generally, the combination of fluoridated adhesive and flowable resin increased the shear bond strength of the bleached dentin. This result may be probably explained by the fact that the fluoridated adhesive system helps in reestablishing the bond strength, which normally is decreased by the use of high concentration bleaching gels. Fluoride positively acts on the demineralized area because it links to the free calcium and phosphate, enabling the remineralization of the dentin, and retards the degradation of this interface, previously unprotected 19. During and after tooth bleaching, the use of topical products containing fluoride results in remineralization and consequently increases the microhardness of the enamel and dentin 3,14.
The analysis of the results obtained in this study lead to the conclusion that the use of fluoridated adhesive associated with a flowable resin is favorable to the restoration of palatal and lingual surfaces of anterior teeth submitted to internal bleaching. The results of this study encourages further clinical studies aiming to increase the bond strength of the dentin submitted to high concentration bleaching agents.
Conclusion
Considering the methodology employed and based on the results obtained, it can be concluded that the combination of fluoridated adhesive and flowable resin increases the shear bond strength of the bleached dentin.
Acknowledgments
We thank to the Brazilian National Council for Research (Pibic/CNPq – grant #136545/2010-6) for the scientific initiation fellowship granted to Nelize Marcelino Pessarello.
References
1. Barbosa CM, Sasaki RT, Flório FM, Bastign RT. Influence of in situ post-bleaching times on resin composite shear bond strength to enamel and dentin. Am J Dent. 2009 Dec;22(6):387-92. [ Links ]
2. Barcellos DC, Benetti P, Fernandes Jr VV, Valera MC. Effect of carbamide peroxide bleaching gel concentration on the bond strength of dental substrates and resin composite. Oper Dent. 2010 Jul-Aug;35(4):463-9.
3. Barros-Matoso F, Souza-Gabriel AE, Furtado-Messias DC, Sousa-Neto MD, Alfredo E. Microhardness of intracoronal dentin exposed to bleaching and fluoride treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011 Nov;112(5):e1-5.
4. Buzalaf MA, Pessan JP, Honório HM, ten Cate JM. Mechanisms of action of fluoride for caries control. Monogr Oral Sci. 2011 Jun;22(1):97-114.
5. Chng HK, Yap AUJ, Wattanapayungkul P, Sim CPC. Effect of traditional and alternative intracoronal bleaching agents on microhardness of human dentine. J Oral Rehabil. 2004 Aug;31(8):811-6.
6. Comlekoglu ME, Gokce B, Kaya AD, Turkum M, Ozpinar B. Reversal of reduced bond strength after bleaching. Gen Dent. 2010 May-Jun;58(3):258-63.
7. Da Costa JB, Mazur RF. Effects of new formulas of bleaching gel and fluoride application on enamel microhardness: an in vitro study. Oper Dent. 2007 Nov-Dec;32(6):589-94.
8. Dahl JE, Pallesen U. Tooth bleaching: a critical review of the biological aspects. Crit Rev Oral Biol Med. 2003 Apr;14(4):292-304.
9. Ferreira EA, Souza-Gabriel AE, Silva-Sousa YT, Sousa-Neto MD, Silva RG. Shear bond strength and ultrastructural interface analysis of different adhesive systems to bleached dentin. Microsc Res Tech. 2010; in press.
10. Joiner A. The bleaching of teeth: a review of literature. J Dent. 2006 Aug;34(7):412-9.
11. Kawamoto K, Tsujimoto Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod. 2004 Jan;30(1):45-50.
12. Khoroushi M, Saneie T. Post-bleaching application of an antioxidant on dentin bond strength of three dental adhesives. Dent Res J. 2012 Jan;9(1):46-53.
13. Kimyai S, Valizadeh H. Comparison of the effect of hydrogel and a solution of sodium ascorbate on dentin-composite bond strength after bleaching. J Contemp Dent Pract. 2008 Feb;9(2):105-12.
14. Lewistein I, Fuhrer N, Churaru N, Cardash H. Effect of different peroxide bleaching regimens and subsequent fluoridation on the hardness of human enamel and dentin. J Prosthet Dent. 2004 Oct;92(4):337-42.
15. Lima AF, Sasaki RT, Araújo LS, Gaglianone LA, Freitas MS, Aguiar FH et al. Effect of tooth bleaching on bond strength of enamel-dentin cavities restored with silorane- and dimethacrylate-based materials. Oper Dent. 2011 Jul-Aug;36(4):390-6.
16. Plotino G, Buono L, Grande NM, Pameijer CH, Somma F. Nonvital tooth bleaching a review of the literature and clinical procedures. J Endod. 2008 Apr;34(4):394-407.
17. Shinohara MS, Peris AR, Pimenta LAF, Ambrosano GMB. Shear bond strength evaluation of composite resin on enamel and dentin after nonvital bleaching. J Esthet Restor Dent. 2005 Jan;17(1):22-9.
18. Shinohara MS, Peris AR, Rodrigues JA, Pimenta LA, Ambrosano GM. The effect of nonvital bleaching on the shear bond strength of composite resin using three adhesive systems. J Adhes Dent. 2004 Mar;6(3):205-9.
19. Shinohara MS, Yamauti M, Inoue G, Nikaido T, Tagami J, Giannini M et al. Evaluation of antibacterial and fluoride-releasing adhesive system on dentin-microtensile bond strength and acid-base challenge. Dent Mater J. 2006 Sep;25(3):545-52.
20. Souza-Gabriel AE, Vitussi LO, Milani C, Alfredo E, Messias DC, Silva-Sousa YT. Effect of bleaching protocols with 38% hydrogen peroxide and post-bleaching times on dentin bond strength. Braz Dent J. 2011 Apr;22(4):317-21.
21. Tam LA, Kuo VY, Noroozi A. Effect of prolonged direct and indirect peroxide bleaching on fracture toughness of human dentin. J Esthet Restor Dent. 2007 Feb;19(2):100-10.
22. Tredwin CJ, Naik S, Lewis NJ, Scully C. Hidrogen peroxide tooth-whitening (bleaching) products: review of adverse effects and safety. Br Dent J. 2006 Apr;200(7):371-6.
23. Uysal TREO, Sagen B, Ustdal A, Akdogan G. Can intracoronally bleached teeth be bonded safely? Am J Orthod Dentofacial Orthop. 2009 Nov;136(5):689-94.
24. Vieira C, Silva-Sousa YT, Pessarello NM, Rached-Junior FA, Souza-Gabriel AE. Effect of high-concentrated bleaching agents on the bond strength at dentin/resin interface and flexural strength of dentin. Braz Dent J. 2012 Jan;23(1):28-35.
25. Wajdowicz MN, Vandewalle KS, Means MT. Shear bond strength of new self-adhesive flowable composite resins. Gen Dent. 2012 Mar-Apr;60(2):e104-8.
 Correspondence:
Correspondence:
Aline Evangelista Souza-Gabriel
Av. Costábile Romano, n. 2.201 – Ribeirânea
CEP 14096-900 – Ribeirão Preto – São Paulo – Brasil
E-mail:aline.gabriel@gmail.com
Received for publication: April 14, 2012.
Accepted for publication: May 18, 2012.