Services on Demand
Article
Related links
Share
RSBO (Online)
On-line version ISSN 1984-5685
RSBO (Online) vol.10 n.1 Joinville Jan./Mar. 2013
ORIGINAL RESEARCH ARTICLE
Evaluation of the inflammatory response of the subcutaneous conjunctive of mice against some endodontic irrigations solutions
Gabriella Guimarães de OliveiraI; Evandro Luiz SiqueiraI; Maria Aparecida NicolettiII; Giulio GaviniI; Marcelo dos SantosI; Fábio Daumas NunesIII
IDiscipline of Endodontics, Department of Operative Dentistry, School of Dentistry of the University of São Paulo – São Paulo – SP – Brazil.
IISchool of Pharmaceutics Sciences, University of Guarulhos – Guarulhos – SP – Brazil.
IIIDiscipline of Oral Pathology, School of Dentistry, University of São Paulo – São Paulo – SP – Brazil.
ABSTRACT
Introduction: Sodium hypochlorite has been used as an endodontic irrigant since 1936, when Walker used it as Sodium Chlorine. Since then it has became the most well acceptable endodontic irrigant. During the last few years many studies have reported the importance of pH control.
Objective: This study evaluates the inflammatory response of some endodontic irrigations solutions in the subcutaneous connective tissue of mice.
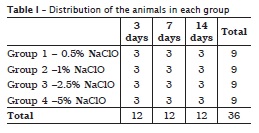
Material and methods: Thirty-six mice were obtained from the Biomedical Sciences Institute at the University of São Paulo. Their backs were divided into four quadrants and each quadrant was injured with an 8 mm punch. Three of these wounds were submitted to differents solutions, while the fourth was used as a control. This experiment was done in triplicate. After the irrigation of the wounds, the mice were sacrificed at 3, 7 and 14 days. The samples were fixed on 10% formalin and histologically analyzed after hematoxylin and eosin staining.
Results: At 14 days all wounds were covered with epithelium with a mild inflammatory infiltrate in the subjacent connective tissue, except for the group that employed 5% sodium hypochlorite at pH 11.
Conclusion: The greatest the pH and concentration of sodium hypochlorite solutions, the most toxic they are.
Keywords: inflammation; sodium hypochlorite.
Introduction
The goals of the root canal preparation are achieved through the cleaning, shaping, and disinfection of the root canal system. Sodium hypochlorite, the most used irrigant worldwide, has an important role in this process contributing with its properties of dissolving organic and antimicrobial tissue.
These characteristics are because of the chloride concentration, pH, temperature of storage, and type of flask, which will account for the performance of the aforementioned characteristics. Notwithstanding, current studies have shown that the endodontist does not know the proper conditions of storage and pH 3,6.
The evolution of the studies resulted in new contributions for the studies on the endodontic microbiology, in which Enterococcus faecalis has an important role, mainly in cases of persisting infections or endodontic retreatments, among which other species, such as: Streptococcus spp., Actinomyces spp., Streptococcus spp., Candida albicans.
The presence of microorganisms within the root canal is one of the main causes of inflammation in the periapical area 21, so that numerous studies have been conducted to confirm the effects of sodium hypochlorite solutions which at high concentrations (2.5% or higher) have been very effective against most of the microorganisms 7,20,25.
Over the years, several studies have demonstrated that sodium hypochlorite at low concentrations are as effective as those at high concentrations as bactericidal agents, therefore reducing the bacterial contamination by either interaction with chelating agents, peroxides or acting alone on biofilms 7,9,11.
Concerning to the dissolution of organic material, several studies have shown that its velocity is directly proportional to the concentration 4,18; however, other studies have disagreed with these conclusions, because they found similar results among different concentrations, pointing out that either temperature or pH accounting for this capacity 24. To make the dissolution more effective, it is fundamental to employ solutions with high values of pH and temperature, because they are capable of denaturing the proteins of the conjunctive tissue1,8.
Among the authors claiming that high concentrations should be used to dissolve organic tissue, Thé et al.28studied the reaction of the subcutaneous conjunctive tissue of rats exposed to sodium hypochlorite at the concentrations of 0.9; 2.1; 4.1 and 8.4 (%) among other solutions, with the aim of determining which sodium hypochlorite concentration should be used in clinical procedures. The authors concluded that the determination of the ideal concentration of sodium hypochlorite should not be based on the intensity of the inflammatory response of the connective tissue, but on the solvent action and antimicrobial effect of these solutions.
On the other hand, accidental leakage of these solutions have been reported to be toxic to the surrounding conjunctive tissue and it may cause hypersensitivity, pain, swelling or even bone necrosis and paresthesia, as well as secondary infections and the possibility of airway obstruction by the swelling 13,14,16,19.22,26.
Although high concentrations of sodium hypochlorite may favor the bactericidal and solvent actions, the control of the inflammatory process also depends on the careful use of chemical agents; otherwise the process may last for longer periods, leading to unfavorable post-operative prognosis.
Thus, the use of solutions at low concentrations has been analyzed both after the cleaning of the root canals and on the connective tissues of the eyes of rabbits. The solutions also caused inflammatory reactions, but they were better supported than the solutions at high concentrations 5,23,27.
The use of sodium hypochlorite solutions needs further scientific studies, because it is known that high concentration solutions in contact with the conjunctive tissue cause undesirable reactions, which is not compatible with the clinical goals of healing and repair. Therefore, this histomorphological study analyzed the inflammatory reaction of the conjunctive tissue of the backs of rats after the use of sodium hypochlorite solutions at different pH and concentration values.
Material and methods
Forty-eight male Wistar rats of (Rattus norvegicus albinus, Rodentia, Mammalia), coming from the vivarium of the Institute of Biomedical Sciences of the University all with weigh ranging from 180 and 220 grams, were used after the approval of the Ethical Committee in Research of the School of Dentistry of the University of São Paulo. The animals were placed into individuals cages, where they received during the experimental phase a balanced ration, proper to their maintenance, ad libitium water and veterinary assistance. Following, they were anesthetized with Francotar® at ratio of 0.1 ml/10000 g of weight associated with Rompum® at 0.05 ml/10000 g of weight, through intraperitoneal route according to the veterinary's recommendation and transferred to a surgical board measuring 30 x 20 cm.
Next, the back of the rat was trichotomized with the aid of a hair cutting machine mounted with a size #0 comb, and complemented with safety razor. The area was then cleaned and disinfected by the friction of gauze embedded in 10% iodopovidone solution.
All 36 rats were divided into four groups, in which 0.5%, 1.0%, 2.5% and 5.0% sodium hypochlorite solutions were used. The wounds were executed with the aid of 8 mm diameter punchs, which were applied in four regions of the backs of the rats: the one at the right side close to skull (wound A), one at the left side (wound B) and other two far from the first punch about 5 cm towards caudal direction, and at the right side (wound C) and left side (wound D). Wound A was used as control while on the wounds B, C, and D the sodium hypochlorite solutions were applied at pH 7, 9 and 11, respectively. Thus, wounds equal in size, shape and deepness were obtained up to the muscle plane.
Sodium hypochlorite solutions were prepared according to Siqueira et al. 24. A 12-15% sodium hypochlorite solution was bought in a chemical house (Casa Bem Te Vi, São Paulo, SP, Brazil) and diluted in distilled water up to the assigned concentrations, confirmed by titrimetry. For the adjustment of the pH values, a pHmeter was used (M12, Horiba, Japan).
The solutions were then stored in amber glass flask under refrigeration until the experiment. Following, with the aid of saline syringe regulated to dispense a drop each 5 seconds, the wounds B, C and D (experimental wounds) received the irrigant solutions during 10 minutes, while the wound A (control wound) received only saline solution. The distribution of the applications is seen in table I.
As the wounds were irrigated, the excess liquid was collected through cannulas coupled to a vacuum bomb placed far from the experimental field about 5 to 10 mm. At the ending, the wounds were washed with 10 ml of saline solution.
Elapsed the times determined for the experiment (3, 7 and 14 days), the animals were euthanized in gas chamber.
After the assurance of the death of each animal, the skin/dorsal muscle fragments were removed with about 4 cm2; three corresponding to the experimental wounds and one to the control wound.
The fragments were placed into plastic flasks containing 10% formalin to fix the tissues properly, embedded in paraffin and cut in a microtome, obtaining histological cuts with 6 μm thick and then stained with hematoxylin-eosin.
Results
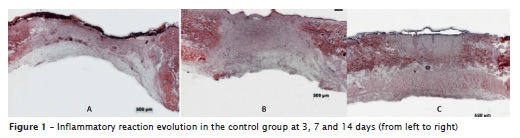
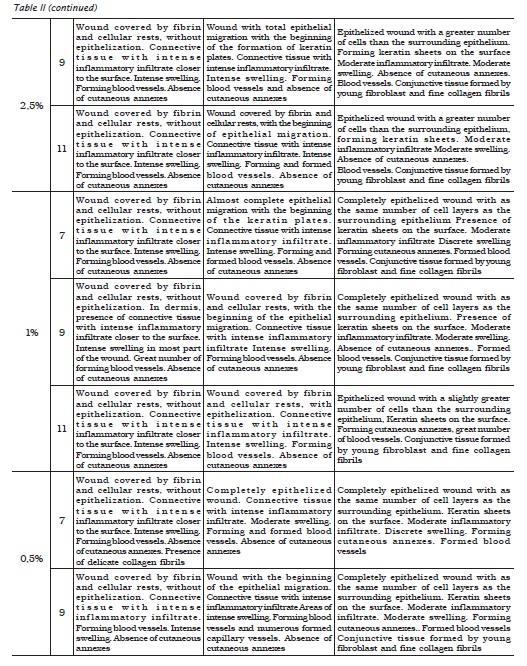
The analysis of the procedures on the control wound, where distilled water was dropped, enabled to verify that at 3 days, the wound was covered with fibrin and cellular rests, without epithelization; granulation tissue with intense inflammatory infiltrate with polymorphonuclear and mononuclear cells closer to the surface and presence of intense swelling in most part of the wound. It was also noted a great number of blood vessels in formation and lack of cutaneous annexes. At 7 days, the histological cut evidenced only the beginning of the epithelial migration as the significant alteration of the first observation time. On the other hand, at 14 days, the analysis revealed a completely epithelized wound, with the same number of cells of the surrounding epithelium, and the presence of keratin films on the surface. The dermis already showed discrete swelling and inflammatory infiltrate, forming and formed cutaneous annexes and the conjunctive tissue still constituted by fine collagen fibrils (figure 1).
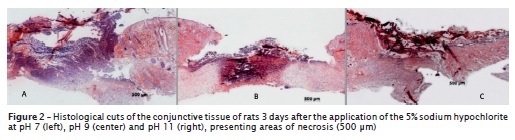
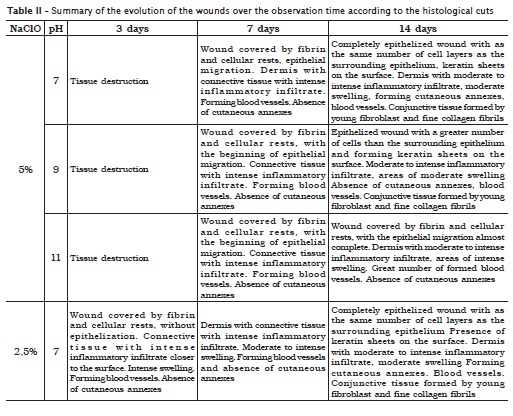
Three days after the procedures, it was verified that all sodium hypochlorite solutions, regardless of their concentration and pH, exhibited absence of epithelium. In dermis, the collagen showed an intense inflammatory infiltrate, swelling and great number of blood vessels, great amount of neutrophils, and lack of cutaneous annexes. The histological cut of the 5% sodium hypochlorite solution, at any pH condition, exhibited an intense area of tissue destruction (figure 2).
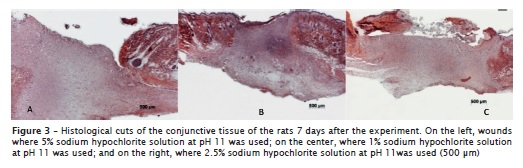
Seven days after the procedures, the histological cuts showed the beginning of the epithelial migrations, except for those receiving 5% sodium hypochlorite solution at pH 11 (figure 3, left), in which the epithelium was absent. In dermis, the presence of a connective tissue with intense inflammatory infiltrate was noted. The swelling was moderated in the analyses where 0.5% and 1.0% sodium hypochlorite solutions at pH 7 were applied; moderate to intense for 1% sodium hypochlorite solution at pH 9; and intense for the other solutions. All the wounds exhibited a great amount of blood vessels, except for 0.5% sodium hypochlorite solution at pH 7 and 9 and 1.0% sodium hypochlorite solutions at pH 7. None histological cut showed the presence of cutaneous annexes. The wounds submitted to 1% sodium hypochlorite solution at pH 7 and 2.5% sodium hypochlorite solution at pH 9 presented formation of keratin plates. The solutions of 5% sodium hypochlorite, at any pH value, exhibited wound covered by fibrin and cellular rests, a phenomenon also noted in those submitted to 1% sodium hypochlorite solution at pH 9 and 11 (figure 3, center) and 2.5% sodium hypochlorite at pH 11 (figure 3, right).
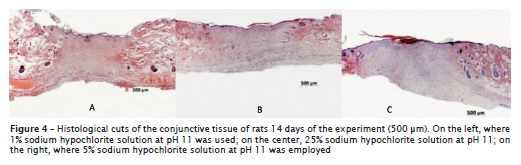
Fourteen days after the procedures, the analysis revealed wounds completely epithelized with as the same number of cell layers as the surrounding epithelium, dermis with moderate inflammatory infiltrate, lack of swelling and presence of cutaneous annexes where all the different solutions had been tested (figure 4, on the left and on the center), except for those submitted to 5.0% sodium hypochlorite solution at pH 9 and 11, in which it was observed the beginning of the epithelial migration, dermis with moderate to intense inflammatory infiltrate, moderate to intense swelling, great amount of blood vessels and absence of cutaneous annexes (figure 4, on the right).

Discussion and Conclusion
The sodium hypochlorite solutions are the most used in Endodontics currently and mainly those at highest concentrations were considered for long time as the most efficient because of their excellent bactericidal capacity and also as solvent of organic material 8.
Researches in microbiology defined the best parameters of the endodontic microbiota, and the facultative anaerobic microorganisms have the main role in the infection of root canals. The sodium hypochlorite solutions are very effective against this microbiota, and the higher the concentrations, the higher the bactericidal effect 25. Still about this aspect, the associations with peroxides or chelating agents provide the increasing of this capacity, respectively by the production of oxygen and removal of the smear layer 7,11,20.
The dissolution, second great contribution of sodium hypochlorite to the chemical preparation of root canal, was already studied by several authors and, almost unanimously, it has been considered that this effect is directly proportional to its concentration 12,18,28.
However, the inadvertent use of sodium hypochlorite solutions has been reported as highly toxic and the literature presents several clinical cases in which swelling reactions and pain are common to these situations 13,14,16,19.
According to Thé et al. 28, the determination of the ideal clinical concentration of sodium hypochlorite should not be based on the intense of the inflammatory response of the conjunctive tissue, but both on the solvent action and bacterial effect of sodium hypochlorite. Notwithstanding Siqueira et al. 24affirmed that pH, not the concentration, is the main responsible for the dissolution capacity, that is, concentration increasing of sodium hypochlorite solutions is little beneficial, on the other hand, the pH increasing is.
The results observed in this study showed the damage caused to the conjunctive tissue, in situations of contact with sodium hypochlorite with very high concentrations and/or pH.
With the application of different solutions, it is observed the presence of inflammatory infiltrate indicating alteration at the first days; this fact can also be confirmed by the swelling and the detection of neutrophils. However, since at 7 days, there was the beginning of the epithelial migration, and at 14 days, the complete epithelization of the tissues, disappearance of the swellings and decreasing of the inflammatory infiltrate for most of the wounds, this confirmed that sodium hypochlorite has a toxic effect consistent with the inflammatory reaction observed in the control group, confirming a low intensity aggression, which is in agreement with Tanomaru Filho et al. 27.
However, in the tissue in which 5% sodium hypochlorite solution at pH 11 was used the following was observed: at 3 days, tissue destruction; at 7 days, absence of epithelial migration; at 14 days, dermis with intense inflammatory infiltrate, intense swelling, great amount of blood vessels and absence of cutaneous annexes, differently from the other wounds, which already showed a great evolution towards repairing.
It could also be verified that the concentration is a key factor for the increasing of inflammation. The increasing of inflammation was directly proportional to the increasing of the chloride concentration of the sodium hypochlorite solutions, except for that at 5%, because of large areas of necrosis, a condition very unfavorable to the necessities and development of the repair process. This study showed that at pH 11 the great amount of sodium hypochlorite was not as aggressive as great concentrations of chloride in the sodium hypochlorite solutions This can be seen by verifying that the sodium hypochlorite solutions at 0.5%, 1.0% and 2.,5% concentrations at diverse pH values did not promote at any moment, drastic reactions such those observed during the use of 5% sodium hypochlorite solutions at pH 11, as swelling reactions or late intense inflammatory infiltrate or even tissue necrosis just at 3 days.
On the other hand Aubut et al. 2 verified that, by buffering the 2.5% sodium hypochlorite solution, the cytotoxicity increased, which was not consistent with the results of this present study, in which the solutions at neutral pH are better acceptable.
Thus, it is clear that the aggressiveness caused by the action of highly concentrated sodium hypochlorite solutions and the solvent effect of these solutions with high pH values, but at low concentrations, is not in agreement with the affirmations of Thé et al. 28. The results of this present study evidenced the need that the choice for sodium hypochlorite solution should also be based on adequate concentration, either by bactericidal requirement, tissue dissolution or tissue compatibility, and at the same time, enable its use at pH 11, since there were not significant inflammatory alterations that may delay the repair process.
References
1. Abou-Rass M, Oglesby SW. The effects of temperature, concentration, and tissue type on the solvent ability of sodium hipochlorite. J Endod. 1981;7(8):376-7. [ Links ]
2. Aubut V, Pommel L, Verhille B, Orsiére T, Garcia S, About I et al. Biological properties of a neutralized 2,5% sodium hypochlorite solution. Oral Med Oral Path Oral Radiol Endod. 2010;109:e120-e5. [ Links ]
3. Ávila LM, Santos M, Siqueira EL, Nicoletti MA, Bombana AC. Análise das soluções de hipoclorito de sódio utilizadas por endodontistas. RSBO. 2010;7(4):396-400400400. [ Links ]
4. Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod. 1992;18(12):605-12. [ Links ]
5. Bombana AC, Paiva JG, Alvares S, Antoniazzi JH. Reação inflamatória do olho de coelho que se segue à instilação de alguns fármacos de uso endodôntico. Rev Assoc Paul Cir Dent. 1974;28(4):216-23. [ Links ]
6. Borin G, Melo TAF, Oliveira EPM. Análise da estabilidade química da solução de hipoclorito de sódio a 1% levando em consideração o local de armazenamento e a quantidade de solução presente no frasco. RSBO. 200008;5(3):7-12. [ Links ]
7. Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18(2):35-4040. [ Links ]
8. Camps J, Pommel L, Aubut V, Verhille B, Satoshi F, Lascola B et al. Shelf life, dissolving action, and antibacterial activity of a neutralized 2.5% sodium hypochlorite solution. Oral Surg Oral Med Oral Path Oral Radiol Endod. 200009;108:e66-e73. [ Links ]
9. Dunavant TR, Regan JD, Glickman GN, Solomon ES, Honeyman AI. Comparative evaluation of Endodontic irrigants against Enterococcus faecalis biofilms. J Endod. 200006;32:527-31. [ Links ]
10. Estrela C, Estrela CRA, Barbin EL, Spanó JCE, Marchesan MA, Pécora JD. Mechanism of action of sodium hypochlorite. Braz Dental J. 200002;13:113-7. [ Links ]
11. Ferrari PHP, Cai S, Bombana AC. Effects of endodontic procedures on enterococci, enteric bacteria and yeasts in primary endodontic infections. Int Endod J. 200005;38:372-80. [ Links ]
12. Junchem CB, Pereira GB, Soares RG, Irala LED, Salles AA, Limongi O. Avaliação da capacidade de dissolução de tecido pulpar bovino pelo ácido tricloroisocianúrico nas concentrações de 1%, 2%, 3% e 4% comparativamente ao hipoclorito de sódio 1%. RSBO. 200008;5(1):34-41. [ Links ]
13. Gernhardt CR, Eppendorf K, Kozlowski A, Brandt M. Toxicity of concentrated sodium hypochlorite used as an endodontic irrigant. Int Endod J. 2004004004;37:272-80. [ Links ]
14. Gursoy UK, Bostanci V, Kosger HH. Palatal mucosa necrosis because of accidental sodium hypochlorite injection instead of anaesthetic solution. Int Endod J. 200006;39:157-61. [ Links ]
15. Hand RE, Smith ML, Harrison JW. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod. 1978;4(2):60-4. [ Links ]
16. Motta MV, Chaves-Mendonça MAL, Stirton CG, Cardozo HF. Accidental injection with sodium hypochlorite: report of a case. Int Endod J. 200009;42:175-82. [ Links ]
17. Murad CF, Sassone LM, Souza MC, Fidel RAS, Fidel SR, Hirata Júnior R. Antimicrobial activity of sodium hypochlorite, chlorhexidine and MTAD® against Enterococcus faecalis biofilm on human dentin matrix in vitro. RSBO. 2012;9(2):143-50. [ Links ]
18. Okino LA, Siqueira EL, Santos M, Bombana AC, Figueiredo JAP. Dissolution of pulp tissue by aqueous solution of chlorehexidine digluconate and chlorhexidine gel. Int Endod J. 2004004004,37(1):38-41. [ Links ]
19. Pontes F, Pontes H, Adachi P, Rodini C, Almeida D, Pinto Jr D. Gingival and boné necrosis caused by accidental sodium hypochlorite injection instead of anaesthetic solution. Int Endod J. 200008;41:267-70. [ Links ]
20. Radcliffe CE, Potouridou L, Qureshi R, Habahbeh N, Qualtrough A, Worthington H et al. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israellii, A. naeslundii, Candida albicans and Enterococcus faecalis. Int Endod J. 2004004004;37:438-46. [ Links ]
21. Ricucci D, Lin LM, Spangberg LSW. Wound healing of apical tissues after root canal therapy: a long-term clinical, radiographic, and histopathologic observation study. Oral Surg Oral Med Oral Path Oral Radiol Endod. 200009;108:609-21. [ Links ]
22. Sermeño RF, Silva LAB, Herrera H, Herrera H, Silva RAB, Leonardo MR. Tissue damage after sodium hypochlorite extrusion during root canal treatment. Oral Surg Oral Med Oral Path Oral Radiol Endod. 200009;108:e46-e9. [ Links ]
23. Simões W, Sampaio JMP, Debelian GJ. Verificação da tolerância tecidual e poder bactericida do hipoclorito de sódio a 0,5% e 1% usados na clínica odontológica. Rev Paul Odontol. 1989;11(4):35-8. [ Links ]
24. Siqueira EL, Santos M, Bombana AC. Dissolução de tecido pulpar bovino por duas composições químicas utilizadas em Endodontia. Rev Pós-grad Facul Odontol Univ São Paulo. 200005;12(3):316-22. [ Links ]
25. Siqueira Jr JF, Machado AG, Silveira RM, Lopes HP, Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal in vitro. Int Endod J. 1997;30(4):279-82. [ Links ]
26. Soares RG, Dagnese C, Irala LED, Salles AA, Limongi O. Injeção acidental de hipoclorito de sódio na região periapical durante tratamento endodôntico: relato de caso clínico. RSBO. 200007;4(1):17-21. [ Links ]
27. Tanomaru Filho M, Leonardo MR, Silva LA, Anibal FF, Faccioli LH. Inflammatory response to different endodontic irriganting solutions. Int Endod J. 200002;35:735-9. [ Links ]
28. Thé SD, Maltha JC, Plasschaert AJM. Reactions of guinea pig subcutaneous connective tissue following exposure to sodium hypochlorite. Oral Surg Oral Med Oral Pathol. 1980;49(5):460-6. [ Links ]
 Corresponding author:
Corresponding author:
Evandro Luiz Siqueira
Rua Guaraiúva, n. 553, apto. 10404 – Brooklin
CEP 0404569-00001 – São Paulo – SP – Brasil
E-mail: pels@usp.br
Received for publication: July 13, 2012
Accepted for publication: August 21, 2012