Services on Demand
Article
Related links
Share
RSBO (Online)
On-line version ISSN 1984-5685
RSBO (Online) vol.10 n.1 Joinville Jan./Mar. 2013
LITERATURE REVIEW ARTICLE
GSTM1 null polymorphism – a possible key for oral carcinogenesis
Tanwar RenuI; Asha R. IyengarI; Kekkeri Sitaram NageshI
IOral Medicine And Radiology, DAPMRV Dental College – Bangalore – India.
ABSTRACT
Introduction: Carcinogenesis is a multistep process and individual risk to development of cancer depends not only on environmental factors or extrinsic exposure to carcinogens but also on genetic susceptibility of an individual. In head and neck cancer, tobacco exposure and alcohol consumption are predominantly the most significant external factors for tumor formation. Individual's susceptibility to cancer may be partly explained by variability in enzymatic activities of metabolic genes. Mutations in genes concerned with production of enzymes for metabolism of tobacco products may lead to increased risk of carcinogenesis with respect to oral mucosa. Therefore variations in the expression of these genes due to heritable genetic polymorphisms might modulate the process of carcinogenesis by altering the exposure levels of tobacco derived carcinogens.
Objective:This non systematic review summarizes current data available on the role of environment gene interaction in form of GSTM1 null polymorphism and oral carcinogenesis.
Literature review: Relevant data was selected in order to summarize the studies conducted on GSTM1 null polymorphism and oral cancer.
Conclusion: Relationship between GSTM1 null polymorphism in oral cancer needs to be established to confirm the role of environment gene interaction in oral carcinogenesis.
Keywords: carcinogenesis; oral mucosa; oral cancer.
Introduction
Oral cancer is one of the 10 most common cancers in the world 16. Tobacco and alcohol exposure are two main etiological agents as in concern to head and neck cancer. Despite the risk of tobacco and alcohol exposure, the majority of patients who smoke or drink alcohol do not develop oral cancer. Factors that influence the tobacco exposed individuals developing a malignancy may thus include a combination of total tobacco exposure and genetic susceptibility of the individual 12.
Environmental carcinogens and certain other endogenous factor (genetic alteration and mutation) interacting in a complex manner can give rise to development of cancer. Three steps are described in the development of cancer namely initiation, promotion and progression. In initiation, a single, somatic cell undergoes non-lethal, heritable mutation. Mutation in the cellular machinery controlling growth or differentiation is an example of the type of genetic change that occurs in initiation 5.
The initiating mutation may provide a growth advantage during the second stage, promotion. In contrast to its normal counterparts, the initiated cell can escape from cellular control mechanisms when responding to external or intercellular signals 5.
Exposure to a tumor promoter will evoke an altered response pattern wherein initiated cells, but not the normal population, are stimulated to grow. The signal to expand clonally can be provided either by direct stimulation of the initiated cell, or as an indirect result of the effects of the tumor promoter on the adjacent normal cells.
Tumor promotion produces relatively benign growths that can be converted into cancer in third stage, malignant conversion. Like initiation, conversion requires genetic alterations in which cellular growth is further deregulated and thus proceeds uncontrolled 5,29.
Mutations induced by oxidants may initiate carcinogenesis; oxidation modification of the genetic material may also participate in the progression of benign to malignant neoplasms. Alteration of the pattern of gene expression by oxidants may function in the stimulation of the initiated cell during tumor promotion. Further, oxidant-induced toxicity in the normal population may facilitate the clonal expansion of the more resistant initiated cell during promotion 2,5,29. The polymorphic genes have subtle effect on cancer risk at individual level but may have a large population impact because the relevant polymorphism may be highly prevalent in a population. These "low-risk" genes can also become important determinants to assess population risk 8.
Various products of these genes are enzymes involved in the activation or degradation of carcinogens/pro-carcinogens. These are termed xenobiotic-metabolizing enzymes (XMEs) found especially in the liver but also in the mucosa of the upper aerodigestive tract, and several are polymorphic and strongly influence individual biological responses to carcinogens. Polymorphic genotypes of these enzymes may serve as genetic biomarkers for susceptibility to certain malignancies and therefore may help predict individual cancer risk 8.
Genetic polymorphisms appear to influence the extent of adduct formation and of p53 mutations. Individual susceptibility to cancer may, therefore, sometimes be explained by a genotype that results in increased carcinogen exposure as a consequence of their carcinogen or pro-carcinogen metabolism. One kind of defense mechanism against development of cancer involves activities of a series of enzymes that metabolize and excrete potentially toxic compounds and repair subtle mistakes in DNA. Head and neck squamous cell carcinoma (HNSCC) is causally associated with tobacco use alone and also in combination with alcohol consumption. Most of the carcinogens present in tobacco and tobacco smoke are converted into DNA-reactive metabolites by cytochrome P450 enzymes and detoxification of these metabolites are performed by glutathione S- transferases and N acetyl transferases in humans. Several of these genes display polymorphisms that could modulate enzymatic activities like activation and detoxification of carcinogens.
Literature review
Most tobacco carcinogens are metabolized via complex enzymatic mechanisms involving both activation and detoxification reactions. Individual's susceptibility to cancer may be partly explained by variability in enzymatic activities of phase I and phase II metabolic genes. Therefore variations in the expression of these genes due to heritable genetic polymorphisms might modulate the process of carcinogenesis by altering the exposure levels of tobacco derived carcinogens.
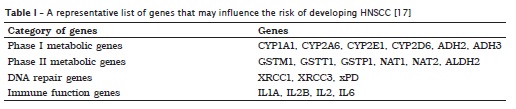
Phase I enzymes [e.g. genes of cytochrome P450 (CYP) family] are generally responsible for conversion of exogenous exposures into carcinogenic metabolites. Most of these metabolites are highly reactive with DNA and responsible for adduct formation and subsequent DNA mutation. Excretion of these intermediate metabolites requires action by phase II enzymes [e.g. glutathione S-transferase (GST) and N-acetyl transferase (NAT) families] to minimize the DNA-adduct formations 17.
Phase II enzymes include glutathione S-transferases (GSTs) and N-acetyltransferases (NATs) that are involved in the detoxification of activated metabolites of carcinogens. The GSTM1 member of the gluthatione S tranferase multigene family catalyzes the conjugation of glutathione to a variety of electrophilic compounds, including carcinogens and cytotoxic drugs 17.
Genetic polymorphisms
GSTM1 and GSTT1
The Glutathione-s-transferases (GSTs) 8,29 are a family of enzymes known to play important roles in the detoxification of several carcinogens found in tobacco smoke. GSTs are dimeric proteins that catalyze conjugation reactions between glutathione and tobacco smoke substrates, such as aromatic heterocyclic radicals and epoxides. Conjugation facilitates excretion and thus constitutes a detoxification step. In addition to their role in phase II detoxification step, GSTs also modulate the induction of other enzymes and proteins involved in cellular functions, such as DNA repair. This class of enzymes is therefore important for maintaining cellular genomic integrity and, as a result, may play an important role in cancer susceptibility.
Role of glutathione-S-transferase in prevention of oral carcinogenesis
All carcinogens are lipophilic and have a tendency to be converted into water soluble hydrophillic compound and can be easily removed from the body through the excretory system. This conversion or detoxification of carcinogens is achieved by the addition of one atom of oxygen to the carcinogenic compound, brought about by the superfamily of Cytochrome P450 (CYP) phase I enzymes. This process of detoxification also leads to the formation of reactive intermediates.
GSTs are a group of phase II enzymes that are primarily involved in detoxifying carcinogenic metabolites, can neutralize the harmful effects of reactive intermediates. The combination of reactive intermediate with an electron deficient DNA base such as guanine forms a DNA adduct. DNA adducts can damage the DNA structure which in turn can lead to the formation of mutants when the DNA replicates. An increased risk of lung cancer, bladder cancer, esophageal cancer and cancers of the oral cavity has been reported in tobacco users.
Of these tumors, oral cancer is unique because tobacco use associated with development of the disease is predominantly chewed. Given equal exposure to the same carcinogens, individuals will vary in their internal processing of the agent depending on genetic background, acquired characteristics and other past or ongoing exposures.
Metabolic activation or detoxification of carcinogenic chemicals to their DNA damaging intermediates appears to be important risk factors. Most of the polycyclic aromatic hydrocarbons first require metabolic activation by Phase 1 enzymes, such as cytochrome p450 gene family to become an ultimate carcinogen, and then subjected to detoxification by Phase 2 enzymes, especially GSTs. The null genotypes that are associated with a lack of enzyme function exist at both these loci and their association with smoking induced cancer has been shown 4.
GST enzymes are coded by at least five distinct loci, known as alpha, mu, pi, theta, and gamma. Also each class includes several genes and isoenzymes. GST M1 products catalyze the conjugation of glutathione to epoxide derivatives of polycyclic aromatic hydrocarbons, the main carcinogens found in the tobacco smoke. GST T1 products are important in the detoxification of naturally occurring monohalomethanes, dichloromethanes, and ethylene oxides. The GST P1 enzyme is widely expressed in the body and detoxifies a variety ofpotential carcinogens, including tobacco smoke-derived substances such as benzo(a)pyrene diol epoxide and acrolein.
Three different polymorphisms have been described at the GSTM1 locus on chromosome 1p13.3.5. The most important polymorphism encodes for a partial gene deletion in GSTM1 (GSTM1 null genotype) resulting in complete absence of GST M1 enzyme activity. The 2 other polymorphisms do not lead to functional differences 29. The frequency of the GST M1 null genotype ranges from 23 to 62% in different populations and is approximately 50% in whites 4. Two loci in particular, GSTM1 (mu-type) and GSTT1 (theta-type) may be of relevance for susceptibility to HNSCC. The GSTM1 and GSTT1 loci have been mapped on chromosomes 1p13.3 and 22q11.2, respectively. Deletion variants of GSTM1 and GSTT1 genes that result in loss of enzymatic activity have been characterized.
Three alleles have been identified at the GSTM1 locus: one is deletion allele and others are GSTM1a and GSTM1b that differ by C-G substitution. This substitution results in replacement of the amino acid, Lys, by the amino acid, Asn, at the position 172 in exon 7 of the protein. This Lys-Asn substitution results in no functional difference between the two alleles. As a result both GSTM1a and GSTM1b alleles are categorized as the positive conjugator phenotype. Two alleles have been identified at the GSTT1 locus – one wild type and the other one deletion-type. Persons who are of the homozygous deletion genotype are categorized into negative conjugator phenotype, while those who carry either one or both of the functional alleles are grouped into positive conjugator phenotype. These two genes have been studied extensively in different populations to find their association with the incidence of tobacco related cancer 3,15,20.
Glutathione S-transferases mu and theta
The GSTM1 and GSTT1 polymorphisms are characterized by the complete loss of enzyme activity in 50 and 20% of Caucasians, respectively. The GSTM1 isoform is responsible, in particular, for the detoxification of nitrosoureas and nitrogen mustards. The GSTT1 isoenzyme is mainly involved in the conjugation of small organic compounds such as dichloromethane and ethylene oxide. Homozygous deletions in the GSTM1 or GSTT1 gene result in missing enzyme activity due to the lack of gene product.
The null variant of GSTT1 appears, therefore, to be involved in the loss of protection against these environmental toxicants. The null genotypes of GSTM1 and GSTT1 are due to several types of alterations in the gene structure occurring either in the coding sequence or in the promoter region, may also be responsible for decreased enzyme activity or expression 3,11,15,18.
The null GSTM1 genotype, with 40-45% of Caucasians nulled at this gene locus, is associated with a lack of enzyme function. Congenital absence of GSTM1 is associated with a higher risk of laryngeal, bladder, colon and gastrointestinal cancer and notably lung cancer in heavy smokers. As for CYP1A1, there are remarkable discrepancies between study results on the GSTM1 null genotype allele distribution of head and neck cancer patients and controls even within ethnic comparable populations.
Discussion
Some suggest an association between the GSTM1 null genotype with oral cavity or head and neck cancer, whereas other working groups did not see any or only a weak relationship between the null genotype and increased cancer risk 1,3,15,18,19,23-25,28.
An investigation was conducted on German population to ascertain the association of polymorphisms of drug-metabolizing enzymes and susceptibility to oral cavity cancer 18. Polymerase chain reaction (PCR)-based analyses were performed on genomic DNA of 94 Caucasian patients in Germany and 92 healthy German controls to determine genotypes of polymorphisms in CYP1A1, GSTM1 and NAT2. The GSTM1 homozygous null genotype occurred more frequently in cancer patients (59.6%) compared to controls (53.3%) but this difference remained insignificant in X2-analysis. Almost identical genotype distributions between cases and controls were found for all three NAT2 acetylator. The results showed no association between CYP1A1, GSTM1 and NAT2 genotypes and susceptibility to oral cavity squamous cancer in the German population studied and suggest that it is unlikely that these polymorphisms cause a predisposition to oral cavity squamous cell carcinoma. In a study based on Japanese population, it was found that those who had null genotypes of GSTM1 had an increased oral cancer risk compared with those who had non-null genotypes of GSTM1, particularly if associated with moderate cigarette smoking 21. In a German population, a study described a significant increase in frequency of the null genotype of GSTM1 in head and neck cancer patients over a control population 10,27. However,these results are limited to laryngeal cancer and do not affect other hypopharyngeal tumors. The discrepancy of the data obtained by oral cavity cancer and laryngeal cancer in the ethnically comparable populations might reflect a tumor-site-related effect of the GSTM1 null genotype.
In a study based on American population in Texas, the glutathione S-transferase genotypes of 186 previously untreated patients with squamous cell carcinoma of the head and neck and 42 healthy controls were determined with polymerase chain reaction (PCR) methodologies and found the absence of both the GSTM1 gene and the GSTT1 gene are associated with elevated odds ratios for head and neck cancer 17,21. The study concluded that genetically determined factors of carcinogen metabolism may be associated with increased risk for head and neck cancer.
A study in Germany was done to investigate concomitant polymorphisms in genes encoding for various detoxification enzymes in patients with head and neck squamous cell carcinoma (HNSCC). In 187 patients with HNSCC and in 139 healthy control subjects, the polymorphisms of cytochrome P450 1A1 (CYP1A1), cytochrome P450 2D6 (CYP2D6), and glutathione S-transferase 1 and (GSTM1, GSTT1) were detected by polymerase chain reaction. No significant association was identified between CYP1A1 and CYP2D6 gene polymorphisms and HNSCC. Patients with laryngeal cancer revealed the GSTM1 null genotype more frequently than did the control subjects. The coincidence of GSTM1 and GSTT1 null genotype was found twice as great in patients as in control subjects. Study concluded that detoxification enzymes are functionally redundant and only the simultaneous deficiency of several detoxification enzymes increases the risk for HNSCC in alcohol- and tobacco-exposed individuals 7,24. In Japanese population a case control study was done with 142 HNSCC patients and 142 healthy control subjects and based on the study results, it was concluded that patients with GSTM1 null polymorphism was present in patients with HNSCC after fewer cigarettes as compared to those with other genotypes and individual differences in polymorphism of GSTM1 gene is one of the important factors in the estimate of risk of oral squamous cell carcinoma at low dose level of cigarette smoking 11,13,30.
Several studies have been done in Indian population to evaluate the relationship between Gstm1 null polymorphism and risk of head and neck cancer. Results have concluded that predisposition to oral cancer is significantly altered in genetic polymorphism involving genes coding for phase II enzymes 3,11,25.
Conclusion
Environment gene interactions, in form of GSTM1 polymorphism and carcinogenesis, share links that can help in prediction of risk for oral cancer development, and use of such markers can aid in prediction of oral cancer susceptibility in exposed individuals.
References
1. Alexandrie AK, Juronen E, Rannug A, Tasa G, Warholm M. Detection and characterization of a novel functional polymorphism in the GSTM1 gene. Pharmacogenetics. 2002;12:613-9. [ Links ]
2. Alin P, Guthenberg C, Jensson H, Mannervik B, Tahir MK, Warholm M et al. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci USA. 1985;82(21):7202-6. [ Links ]
3. Alldersea J, Bockmuhl U, Jahnke V, Jones PW, Hayes JD, Matthias C et al. Polymorphism in cytochrome P450 CYP2D6, CYP1A1, CYP2E1 and glutathione S-transferase, GSTM1, GSTM3, GSTT1 and susceptibility to tobacco-related cancers: studies in upper aerodigestive tract cancers. Pharmacogenetics. 1998;8(2):91-100. [ Links ]
4. Amagasa Y, Yamashiro M. Oral leukoplakia related to malignant transformation. Oral Science International. 2006;3(2):45-55. [ Links ]
5. Arzani D, Boccia S, Cadoni G, Digiannantonio P, Feo E, Gallì P et al. A review of genetic epidemiology of head and neck cancer related to polymorphisms in metabolic genes, cell cycle control and alcohol metabolism. ACTA Otorhinolaryngologica Italica. 2012;32:1-11. [ Links ]
6. Bartsch H, Nair U, Risch A. Genetic polymorphisms of CYP gene, alone or in combination as a risk modifier of tobacco related cancers. Cancer Epidemiol Biomarker Prev. 2009:3-28. [ Links ]
7. Beckett GJ, Bell D, Hayes JD, Hayes PC, Howie AF. GlutathioneS-transferase isoenzymes in human bronchoalveolarlavage: a possible early marker for the detection of lung cancer. Carcinogenesis. 1990;11(2):295-300. [ Links ]
8. Bowman ED, Butkiewiez D, Seker H, Rusin M, Hedayati M, Grossman L et al. Functional significance of xPD polymorphic variants: attenuated apoptosis in human lymphoblastoid cells with the xPD 312 asp/as genotype. Cancer Res. 2001;61:7430-4. [ Links ]
9. Califano JA, Grumbine FL, Mithani SK, Mydlarz WK, Smith IM. Molecular genetics of premalignant oral lesions. Oral Diseases. 2007;13:126-33. [ Links ]
10. Carretero P, Cuchi A, Lafuente A, Pujol F, Villa JP. Human glutathione S-transferase mu (GST mu) deficiency as a marker for the susceptibility to bladder and larynx cancer among smokers. Cancer Lett. 1993;68(1):49-54. [ Links ]
11. Chern HD, Chiang CP, Chien YC, Chuang J, Hung HC, Kuo YS et al. Genetic polymorphisms of CYP2E1, GSTM1, and GSTT1; environmental factors and risk of oral cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(11):901-5. [ Links ]
12. Clayman GL .Glutathione S-transferase genotypes as risk factors for head and neck cancer. Am J Surg. 1995;170:499-501. [ Links ]
13. Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama Y, Nebert DW, Nelson DR et al. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991;10(1):1-14. [ Links ]
14. Duarte G. GSTM1 polymorphism and oral leukoplakia. J Oral Pathol Med. 2006;35:202-5. [ Links ]
15. Fryer AA, Strange RC. The glutathione S-transferases: influence of polymorphism on cancer susceptibility. IARC Sci Publ. 1999;148:231-49. [ Links ]
16. Gronau S. Gene polymorphisms in detoxification enzymes as susceptibility factor for head and neck cancer? Otolaryngol Head Neck Surg. 2003;128:674-80. [ Links ]
17. Guyton KZ, Kensler TW. Oxidative mechanisms in carcinogenesis. Br Med Bulletin. 1993;49:523-44. [ Links ]
18. Hahn M. Genetic polymorphisms of drug-metabolizing enzymes and susceptibility to oral cavity cancer. Oral Oncology. 2002;38:486-90. [ Links ]
19. Hallikerimath S, Kale AD, Shukla D, Vivekanandhan S, Venkatakanthaiah Y. Genetic polymorphism of drug metabolizing enzymes (GSTM1 and CYP1A1) as risk factors for oral premalignant lesions and oral cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156:1-7. [ Links ]
20. Hao K, Shi R, Zhang ZJ. Glutathione S-transferase M1 (GSTM1) and glutathione S-transferase T1 (GSTT1) null polymorphisms, smoking, and their interaction in oral cancer: a HuGE review and meta-analysis. Am J Epidemiol. 2011;173:847-57. [ Links ]
21. Hamajima G. Triplex polymerase chain reactions with confronting two-pairprimers (PCR-CTPP) for NQO1 C609T, GSTM1 and GSTT1 polymorphisms: a convenient genotyping method. Asian Pacific Journal of Cancer Prevention. 2003;4:67-70. [ Links ]
22. Hayashi SI, Kawajiri K, Nakachi K, Watanabe J. PCR detection of an A/G polymorphism within exon 7 of the CYP1A1 gene. Nucleic Acids Res. 1991;19(17):47-57 . [ Links ]
23. Hayes J, Pulford D. The glutathione-S-transferase supergene family: regulation of GST and the contribution of the contribution of the isoenzymes to cancer chemoprevention and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445-600. [ Links ]
24. Hiyama T, Tanaka S, Yoshihara M. Genetic polymorphisms and head and neck cancer risk. Int J Oncol. 2008;32:945-73. [ Links ]
25. Izumo T, Sato M. Genetic polymorhism in drug metabolising enzymes and suscepitibility to oral cancer. Carcinogenesis. 1999;20(10):1927-31. [ Links ]
26. Konig-Greger D, Rettinger G, Riechelmann H. GSTM1 gene polymorphism in patients with head and neck tumors. Laryngorhinootologie. 2000;79(6):341-4. [ Links ]
27. Petersen PE. Oral cancer prevention and control – the approach of the World Health Organization. Oral Oncol. 2009;45(4-5):454-60.
28. Rebbeck T. Molecular epidemiology of the human glutathione – S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 1997;6:733-43.
29. Sciubba JJ. Oral cancer and its detection, history taking and the diagnostic phase of management. J Am Dent Assoc. 2001;132:12s-8s. [ Links ]
30. Unal A. Glutathione S-transferase M1, T1, and P1 gene polymorphism in laryngeal squamous cell carcinoma. Am J Otolaryngol. 2004;25:318-22. [ Links ]
 Corresponding author:
Corresponding author:
Tanwar Renu
SGT Dental College, Hospital and Research Institute – Village Budhera – Gurgaon – Haryana – India
E-mail: renu_bds7@yahoo.co.in
Received for publication: April 12, 2012
Accepted for publication: July 30, 2012