Services on Demand
Article
Related links
Share
RSBO (Online)
On-line version ISSN 1984-5685
RSBO (Online) vol.10 n.2 Joinville Apr./Jun. 2013
ORIGINAL RESEARCH ARTICLE
Evaluation of active chlorine releasing of sodium hypochlorite during seven days, stored at different temperatures
Gladyvam Rabêlo BraittI; Evaldo de Almeida RodriguesI; Carlos Eduardo da Silveira BuenoII; Antonio Henrique BraittI
I Program of Specialization in Endodontics of the Institute of Health Sciences, Funorte/Soebras, Núcleo Ilhéus – Ilhéus – BA – Brazil
II São Leopoldo Mandic Center of Post-Graduation – Campinas – SP – Brazil
ABSTRACT
Introduction: The sanitation of the canal system through irrigation/aspiration, at the changing of the endodontic instruments aims to the excised material, removal of microorganisms and the cleaning of the walls of the canals. One of the substances used in endodontic treatment of root canals, sodium hypochlorite, used at different concentrations of active chlorine and pH, has gained popularity due to its physical chemical properties.
Objective: To analyze the active chlorine content of sodium hypochlorite solution at 6.0%.
Material and methods: 80 samples were obtained from two litres of sodium hypochlorite at 6%, obtained at every hour: one liter was stored at room temperature and one liter at refrigerated environment, between the morning and afternoon shifts, except on Saturday and Sunday. The free residual chlorine was determined, using as variables: the temperature, length of time in storage and the handling of the substance in the Endodontic clinic for seven days. The data obtained were submitted to analysis of variance according to the linear regression model to test the effect of time of storage condition and interaction between the main effects on the chlorine content of the stocked solution (p < 0.01).
Results: The results showed that the sodium hypochlorite solution is quite unstable, with considerable loss of active chlorine (58.33%), depending on the storage conditions and storage time and temperature. The temperature interferes in the free residual chlorine concentration contained in sodium hypochlorite solution, that is, the higher the temperature, the lower the lifetime of free residual chlorine in sodium hypochlorite solutions.
Conclusion: Storage in refrigerated environment proved to be the best option to avoid the marked loss of active chlorine in sodium hypochlorite of concentration at 6%.
Keywords: Endodontics; sodium hypochlorite; irrigating substances.
Introduction
The irrigating substance should make ease the removal of the organic and inorganic remnants (including microorganisms), promoting the root canal and dentinal tubules sanitation simultaneously to the absence of damages to periapical tissue 4.
Hypochlorite is bleaching agents commonly used to bleach textiles or papers and to disinfect the solutions. The solution normally contain 10 to 15% of available chlorine (equivalent to 100 to 150 g/L), but rapidly loses its force during the storage. Because the solution is very affected by the heat, light, pH and heavy metals, the solution needs to be regularly controlled.
Although variations exist regarding to the concentration recommended, the active principle of hypochlorite is the same. It is a very unstable substance and this instability have been already highlighted by Dakin 6 and confirmed by several studies conducted 8,11,12.
Introduced in Dentistry by Barret 1, in 1917, sodium hypochlorite (NaOCl) is a very efficient irrigating solution of the root canals because of its properties such as deodorant, bactericidal and solvent of the tissues 2,10.
Because sodium hypochlorite is the irrigating substance with the best physical-chemical property among the armamentarium of substances used in the cleaning and shaping of root canals, it is the irrigating agent most utilized in Endodontics.
Gomes et al. 8, by analyzing the concentration of active chlorine in sodium hypochlorite solutions at dental offices, concluded that most of the solutions were the Dakin's liquid which however showed the greatest variation of loss of active chlorine. The storage most found was in white plastic flasks stored at the counter or cabinet. The Milton's solution and chlorinated soda was the most stable. The pH of the samples was inside the ideal values for stability; however, most of the professionals did not use the real concentrate they believe to be using. Fabro et al. 6 observed that the studies conducted in Brazil have demonstrated that the dentists have preferred sodium hypochlorite solutions in concentrations at around 0.5 to 1.0%; however, because of the constant chemical reaction, there is the loss of active chlorine and at the moment of its use they are very weaker and do not exhibit the desired action.
Braitt et al. 3, referring to the rotary instrumentation of the root canal treatment, they emphasized the necessity of a copious irrigation concurrent with the use of the instruments otherwise organic and inorganic material (smear layer) and putrefaction material and microorganisms will stay adhered on root canal wall, which prevents the sanitation of the root canal system and impedes a complete obliteration of the canal during the endodontic obturation.
Most of the studies regarding the temperature and storage time of the sodium hypochlorite employed in the endodontic clinics was performed with the liquid stored in different temperatures; notwithstanding, the samples for evaluation of the sodium hypochlorite stability were carried out at relatively longer intervals once it was not considered that in the endodontic clinic this substance is handled, in mean, eight times per day with interval of two hours between the morning and afternoon shifts and pause of generally two days per week (Saturday and Sunday).
Aiming to contribute for the study of the temperature influence on the releasing of chlorine which can lead to the decrease of the free chlorine and consequently to the loss of the bactericidal power, this present study was conducted to establish which would be the storage option more suitable for the maintenance of the active chlorine from 6% sodium hypochlorite used in the endodontic clinics, by analyzing the content of this solution.
Material and methods
The concentration of active chlorine of sodium hypochlorite solutions at 6.0% was determined by titration, iodometry method, through the determination of the free residual chlorine. The following variables were utilized: storage time and the substance handling in the endodontic clinics for seven days, by observing the number of the daily collection, pause between the morning and afternoon shifts as well as the lack of handling during the Saturday and Sunday.
The reagents and solutions employed in the methodology execution for samples A and B comprised a set for 100 tests for lixivium (HI 3843, Hanna Instruments, São Paulo, Brazil), with gamma specification from 50 to 150 g/L as chlorine (C12), smaller increment of 5 g/L (0.5%) as chlorine (C12), with size of the sample of 1 ml (figure 1).
It is also used a universal indicator set of pH 1-14 with 200 bands (J Prolab Ind. e Com. de Produtos para Laboratório Ltda., São José dos Pinhais, Brazil).

Stages of the procedure
Sample A – environment temperature
In about 70-75 ml of distilled water within an Erlenmeyer flask, six drops of the reagent HI 3843A-0 were added, carefully agitated for mixing. Following, to obtain a pH lower than 3, a package of the reagent HI 3843B-0 was dissolved within this mixture and the pH was measured in the Erlenmeyer flask with the aid of litmus paper.
In a plastic flask, 25 ml of sodium hypochlorite from sample A (environment temperature) was added, with the measurement of the temperature at the moment of the collection. From this sample, with the aid of a syringe, 1 ml of sodium hypochlorite was collected and added to the Erlenmeyer flask. The presence of the sodium hypochlorite makes the solution to be dark orange. Slowly, drops of titration reagent HI 3843C-0 were added, and after each drop the solution was agitated. Each drop was counted until the solution changing from dark orange to colorless solution, as seen in figure 2.

Sample B – refrigerated environment
The same procedure steps executed in sample A were adopted, and 25 ml of hypochlorite from sample B (refrigerated environment) were added within the plastic flask, with temperature measurement at the refrigerated environment at the moment of the collection.
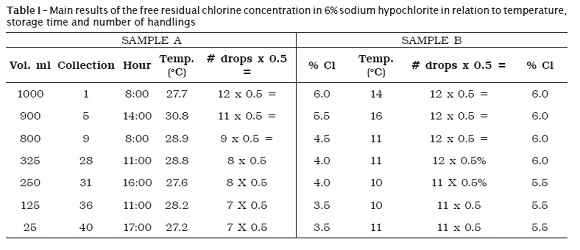
To obtain the chlorine concentration at % of the sample, the number of drops of the reagent C of the titration was multiplied by 0.5 to make acolorless solution, according to the manufacturer's instructions, in which # of drops x 0.5= % of chlorine.
The calculations were performed with the use of the procedures of SAS system– INSTITUTE Inc (The SAS system – release 9.2.SAS Insitut82ute Inc. Cary:NC. 2011.2008). The data were statistically analyzed through analysis of variance with level of significance of 5%.
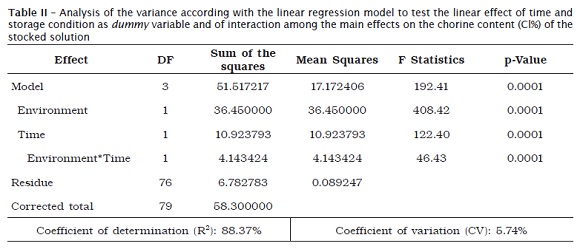
The analysis of the variance of the effects planed as determinants of the variations of the chlorine content of the solutions studied is seen in table II.
Results
This present study was conducted to evaluate the alterations observed over time of the solutions stored in two conditions: environment and refrigerator. In table I are seen the main data regarding to the evaluations of the sodium hypochlorite in relation to the factors of temperature variation and storage time, whose graphic representation is shown in graph 1.
The tests from each effect separately strongly indicates (p < 0.01) that all are significant, i.e., both the time and the storage condition and the interaction among the main effects are not casual. The regression model is shown as graph.
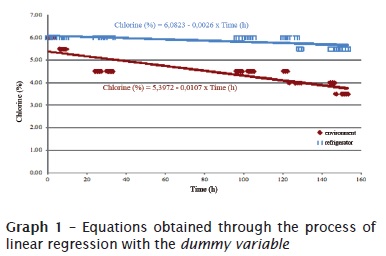
For the execution of the analysis of the data and obtainment of the subsides to the decisions inherent to the research, it was adopted to the technique of the analysis of the linear regression with the dummy variable (storage condition), with a continue variable (time) and the interaction between them. Such technique enables the estimation of the parameters of a model that allows the prediction of the variation of the amount of chlorine and the testing of the significance of the effects listed in the model.
Discussion
Since its introduction in Endodontics, sodium hypochlorite always has a detached position during the preparation phase of the root canal, once in addition to combine with several other substances in the search for the greatest bactericidal effect, it has qualities such as the tissue compatibility, tissues solvency, increase of the dentinal permeability and root canal cleaning. Most of the studies regarding to the temperature and storage time of the sodium hypochlorite employed in the endodontic clinics were conducted with the liquid stored at different temperatures; however, the collections for the evaluation of the sodium hypochlorite stability were performed in relatively longer intervals, once it was not considered that in endodontic clinics this substance is handled, in mean, eight times per day with interval of 2 hours between the morning and afternoon shifts, with pause of generally two days per week (Saturday and Sunday).
Also Clarkson et al. 4 pointed out the necessity that sodium hypochlorite be stored in opaque and closed flasks because the constant opening of the flasks causes a greater loss of chlorine. Considering the necessity of the constant opening of the hypochlorite solution stored in the endodontic clinics and that the hypochlorite solution stored in and refrigerated environment (between 9 and 13oC) only started to lose active chlorine at the collection number 31 (750 ml), it is recommended that, for safe, the product be stored in flasks of 500 ml.
According to the British Pharmacopeia Commission, the titration process, also called iodometry, it is the most efficient and reliable method to determine the concentration of the active chlorine.
Through titration, iodometry method, it is possible to determine the amount of active chlorine in a sodium hypochlorite solution. The iodine moves the active chlorine present in the solution at the proportion of 1 mole to 1 mole. When sodium thiosulfate is added to the solution, an oxi-reduction reaction of iodine occurs so that it is possible to determine the amount of this substance present. Thus, which is being titrated is the iodine, but as it is present at the same proportion than chlorine, its concentration is easily determined. The chlorine available is related to the chlorine released by the action of the acid diluted in the hypochlorite.
According to the manufacturer's instruction, the hypochlorite solution is treated with potassium iodide and highly acidified. The amount of iodine generated is equivalent to the chloride from the sample. The iodine concentration is then calculated by the titration of the thiosulfate ions which reduces the iodine back to the iodide ions.
Although the aim of this present study had been to analyze the content of the sodium hypochlorite, through the determination of the free residual chloride, using variables as temperature, storage time and the substance handling in the endodontic clinics, it is worth highlighting that, by analyzing the calculus to obtain the concentration with the aid of the kit, it was verified that the minimum value that can be measured is 0.5%, since there is not possible to use less than a drop. This fact demonstrates that with the calculation, in none of the hypotheses would be possible to achieve values lower than 0.5. Such fact displays the possibility of an experimental error of the method at the rate of ±0.25%; for example, in a result of 0.5%, the measurement can be 0.25% or 0.75%, values which can be very large in the variation of the concentration used in Endodontics.
Conclusion
Based on the analysis of the results, it can be concluded that:
• Sodium hypochlorite is a very unstable solution, with considerable loss of active chlorine, depending on the storage conditions and time;
• The storage temperature interferes in the content of free residual chlorine within the sodium hypochlorite. The higher the temperature, the shorter the life span of the free residual chlorine within the sodium hypochlorite; the storage in refrigerated environment was the best option to avoid the marked loss of active chlorine from 6% sodium hypochlorite.
References
1. Barret MT. The Dakin-Carrel antiseptic solution. Dental Cosmos. 1917;59(4):446-8. [ Links ]
2. Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod. 1992;18(12):605-12. [ Links ]
3. Braitt AH, Cunha RS, Martin AS, Bueno CES. Evaluation of cleaning efficacy of a nickel-titanium rotary system, with or without 17% EDTA passive ultrassonic activation: a scanning electron microscopic study. RSBO. 2012;9(1):38-43. [ Links ]
4. Clarkson RM, Moule AJ, Podlich HM. The shelf-life of sodium hypochlorite irrigating solutions. Aust Dent J. 2001;46(4):269-76. [ Links ]
5. Dakin HD. On the use certain antisseptic substances in the treatment of infected wounds. Brit Med J. 1915;2(28):318-20. [ Links ]
6. Fabro RMN, Britto MLB, Nabeshima CK. Comparação de diferentes concentrações de hipoclorito de sódio e soro fisiológico utilizados como soluções irrigadoras. Odontol Clín Cient. 2010;9(4):365-8. [ Links ]
7. Gambarini G, De Luca M, Gerosa R. Chemical stability of heated sodium hypochlorite endodontic irrigants. J Endod. 1998;24(6):432-4. [ Links ]
8. Gomes MCP, Britto MLB, Nabeshima CK. Análise da concentração de cloro ativo em soluções de hipoclorito de sódio encontradas em consultórios odontológicos. Rev APCD. 2010;64(2):150-4. [ Links ]
9. Grossman LI, Maiman BW. Dissolution of pulp tissue by chemical agents. J Amer Dent Ass. 1941;28:223-5. [ Links ]
10. Johnson BR, Remeikis NA. Effective shelf-life of prepared sodium hypochlorite solution. J Endod. 1993;19(1):40-3. [ Links ]
11. Pécora JD, Guerisoli DMZ, Silva RG. Shelf-life of 5% sodium hypochlorite solutions. Braz Endod J. 1997;(2):43-5. [ Links ]
12. Piskin B, Tükün M. Stability of various sodium hypochlorite solutions. J Endod. 1995;21(5):253-5. [ Links ]
 Corresponding author:
Corresponding author:
Gladyvam Rabêlo Braitt
Av. Aziz Maron, n. 1.117, sala 703 – Jardim União
CEP 45605-415 – Itabuna – BA – Brasil
E-mail: gladybraitt@hotmail.com
Received for publication: November 5, 2012
Accepted for publication: December 14, 2012